The subsequent study originally appeared in the Volume of the 7th Symposium (Side Tracks in Evolution) of the Matter Evolution Subcommittee of the Geonomy Scientific Committee of the Hungarian Academy of Sciences. The Symposium was held in 1996, but because of complications the Volume appeared only in 1998, as B. Lukács & Sz. Bérczi (eds.): Side Tracks in Evolution; KFKI-1998-07/C. The article was pp. 20-41.
However later the 4 authors slightly rewrote the text for journal publication and definitely omitted some hand-drawn Figures. I worked here from that variant, except that I had to digitalize even some of the remaining Figures; this may explain some slight technical problems with Figures, but they will do even in their present forms. Unfortunately this variant was not published: we submitted it to a journal, got a lengthy referee report about modifications (which we have made) and then one of ours told to the others that the journal expired meantime.
Two years later I discovered traces of later publications in the respective Journal and asked the informant what was the reason for the misinformation; I got an angry but indefinite answer, and the matter remained in that stage. I do not understand at all, what had happened.
But why not to put it on Internet? I think it was a honest and interesting work in Evolution; in that time I was the President of the Matter Evolution Subcommittee and another author was Secretary, while now I am still the President and another author is a Member. So enjoy it if you can.
Now I changed the text only as in a galley-proofing; and I inserted 5 superscript Notes (from A to E in this Lucida Calligraphy) where even I would write something differently after 7 years. The Footnotes will come at the very end.
So let the text come.
************************
MONOTREMES: ABOUT THE ROLE OF PERMOTRIASSIC AND JURA-CRETACEOUS BOUNDARIES IN THE ATHERIAN-THERIAN DIVERGENCE
Bérczi Sz.1, Holba Á.2, Lukács B.2, and Papp É.3
1 Department of General Technology, Cosmic Matter Research Group, Eötvös L. University, H-1117 Budapest, Pázmány Péter sétány 1a, Hungary
2 Central Research Institute for Physics RMKI, H-1525 Bp. 114. Pf. 49, Hungary
3 Department of Geology, University of Perth, 6009 WA Nedlands, Australia
ABSTRACT
The three recent Australian egg-laying "mammals" stand quite apart from the bulk of mammalians. In some sense they are "archaic", but some of their distinctive features are not at all plesiomorphic. It is not yet a settled question if the last common ancestor of them and the others had reached the mammalian grade, and the way of their locomotion is more conservative than of some of the "first mammals" from basal Jurassic. It seems that egg-layers represent an evolutionary path which was roughly parallel with ours, but on which the evolution was slower. If so, this is a good example for highway vs. sidetrack relation in evolution.
Keywords: Monotremata, Perm-Triassic boundary, tritheledonts, monophylecy.
THE PROBLEM STATED
At the first half of the last century Australia revealed a strange group of mammals. The duckbill ("platypus") and the spiky anteater ("echidna") seemed to interpolate between mammals and birds (or mammals and reptiles): they have beaks instead of teeth; lay eggs but give milk to the offshot; there are milk glands but not teats; they are warm-blooded but only hardly so; the shoulder girdle is quite reptilian with an independent coracoid and horizontal humerus with sprawling gait; there is a reptilian (and avian) fashioned cloaca (hence the name Monotremata or "one-holed") and so on. (For a general orientation see e.g. Chaps 15 & 16 of WALTON & RICHARDSON 1989). There were two alternative explanations for these important differences:
1) The monotremes may have evolved on the general mammalian pathway, but more slowly in the isolated Australian environment and therefore they may resemble now our general status some dozens of million years before, nearer to the reptile/mammal split; or
2) They may have been following a different evolutionary path.
In Case 1) the monotremes would be simply living relicts. In Case 2) the monotreme path is obviously a sidetrack compared to the general mammalian evolution. Although natural sciences rather avoid qualitative statements, it is hard to deny that in our age the monotremes are less successful than ourselves. A quantitative argument is 3 recent monotreme species compared to the many thousands of placentals and marsupials. In the same time, the monotreme evolution seems to have been fairly parallel to ours (albeit slower). While there are independent monotreme innovations (e.g. the molar structure), many of the specific monotreme characteristics are not independent innovations but archaisms. So monotremes vs. other recent mammals seem a good example for highways and sidetracks of the evolution, and we learn something by observing the details of mammalian evolution.
There is a Yes or No question in these processes, and Yes or No type questions are often fascinating. The question is: Are Mammals monophyletic or polyphyletic? Obviously the old Class Mammalia was not a clade but a grade, and therefore there is a possibility that the class is not monophyletic, therefore not a valid taxon in "natural taxonomy". About the classical taxa of Vertebrates now we know that Amphibia are polyphyletic. We do not discuss here the mono/polyphylecy of Reptilia (cf. HUEHNE 1956), but independently of that more than one "reptile" lineage may have crossed the mammalian demarcation line, and the mono/polyphyletism is still open for recent mammals. So one may hope that by investigating the two ways of mammalian evolution it will be possible to get an answer to the question: have already been the monotremes mammals when splitting from the other "mammals"?
As we shall see at the end, the answer is still uncertain and a matter of definition. However, it will be interesting to see, how.
The next 2 parts will give nomenclature and definitions, very necessary in the present case, of which "common sense" tells practically nothing. Then we briefly recapitulate the main points of the evolution of the clade Synapsida, containing all mammals together with their reptile ancestors and close relatives. Afterwards we give some partly uncertain, information about the environmental conditions during the therapsid/mammal transition, which ones may or may not have driven the transition.
Since mammals vs. reptiles are defined and told apart via very various and sometimes arbitrary criteria, almost always based on recent groups, we give a four dozen criteria, which lead to contradictory results when applied on some therapsids and monotremes. Then the results of a recent, very important, DNA-hybridization measurement for the age of the last common ancestor will be summarized and reanalysed, which, however, again lead to self-contradictory conclusions. The lend of the main text tries to summarize the situation. 4 parts of Appendices give more insight into some critical points.
DEFINITIONS BY CHARACTERISTIC FEATURES AND THEIR CORRELATIONS
If we want to make any statement whether the last common ancestor of monotremes and other recent mammals was still reptile or already mammal ("are the present mammals mono- or diphyletic?"), then we first must define the difference between mammals and reptiles, in such an operative way which may be applied on fossil remnants.
The classical and very transparent definition of mammals vs. reptiles comes from Aristotle, Linné, and from common sense. It goes somehow as: mammals give birth to living kids, in contrast reptiles lay eggs. Mammals give milk to the offshot via teats while reptiles have no teats and do not feed the young. Mammals are covered by hair while reptiles are by scales. Mammals are warm-blooded with constant temperature while reptiles are poikilotherm.
Obviously there are serious problems with this definition. According to the first criterion monotremes are not mammals. The second criterion is doubtful, since monotremes have milk glands but not teats; indeed they have no mammae i.e. udders, and it is not easy to classify an animal without mammae as mammal (except if it had had it but have lost; but that is not the case now). The third criterion would classify monotremes to mammals; but remember that some insectivores still have vestigial scales at the end of the tail, and the "placental" Manis has quite developed scales. Finally, the fourth criterion prefers monotremes to be mammalian, but how constant should be a temperature to call the animal homeotherm?
Anyway, there may always be problems with definitions using more than one criteria. It is logically possible to imagine a warm-blooded animal with fur but without milk. (Note that birds are as homeotherm as mammals.) Such an animal may or may not have existed; and then which is the decisive criterion? Good classification can be based only on a single criterion (or on a hierarchy of criteria), to which the only possible answer is Yes or No. No doubt, fur and milk are absolutely correlated on living animals, but as we see, viviparosity and fur are not.
As it will be seen, fossils connect quite smoothly one branch of extinct reptiles and mammals; but milk and egg do not fossilize. So the next idea was to find a bony structure strongly correlated with "mammalness". The suggested decisive characteristics became the articulation of the lower jaw and the skull. In all recent reptiles the only connection is the articulare and the quadratum, while in all recent mammals the dentale to the squamosum, while the articulare and the quadratum have moved to the middle ear, where they are now the malleus and the incus. In addition, the reptilian lower jaw consists of several bones (at least the dentale, angulare and articulare), while the general belief is that the mammal lower jaw is the single dentale. (However marsupials may perhaps have the angulare too (GÉCZY 1989).)
The difference is important, and for some time it was believed that a "macroevolutionary jump" happened between the Art/Q and D/Sq connections. This, however, is no more the situation, the discovery of Diarthrognathus made the picture much subtler. Diarthrognathus possessed double articulation between skull and lower jaw: articulare to quadratum and dentale to squamosum. So its articulation was synchronously "reptilian" and "mammalian"; there was no "macroevolutionary jump" at this point. Now, using the above definition in the direction "having the D/Sq joint", Diarthrognathus is mammal, using backwards "no more Art/Q jaw-skull hinge", it is not. As seen later, the transition lasted from Upper Triassic to at least the Upper Jurassic, some 50 Ma. So the definition must be refined or changed. Specifically so, because the original definition was not in any direct connection with "being mammal" in the usual sense; its only advantage was that it was believed to be a Yes or No question. The end of the Art/Q jaw hinge may have been a milestone because then hearing improved substantially; but it would be strange to define mammals through the improved auditory sense and nothing else.
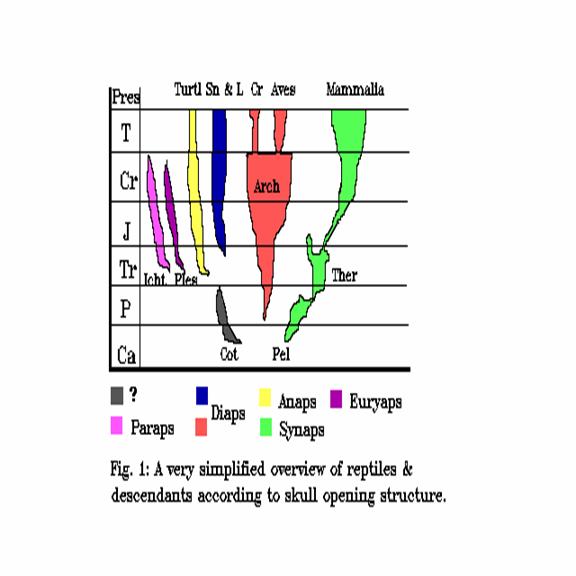
We stop here and switch to taxonomy because some problems are burdened by nomenclature. It turned out that some 300 Ma ago, in Upper Carboniferous, the primitive stem reptiles branched into several groups by lightening their skull structures. The original skull structure was closed except 3 openings for the eyes (left, right and parietal) and one or two for the nose. A group (Anapsida) retained the closed skull, and is now represented by the turtles. The other groups opened up one or two pairs of lateral windows, at various locations (see Figure 1), and according to present consensusA, the original structure is recognisable even if modified later. All the recent reptiles, except for turtles, belong to Diapsida, two windows above each other in the temporal region. Dinosaurs belonged also to Diapsida, and so do their non-reptile descendants, the birds.
|
|
Figure 1: The main reptile groups according to side openings.
All other branches are extinct as reptiles, but one branch left non-reptilian descendants behind, the branch Synapsida (one window on both sides just behind the lateral eyes). All mammalians are of synapsid origin, and no mammal exists which were not. In fact, mammals may be called also synapsids, although in later reptilian synapsid evolution the arch between the window and the eye opening vanished; Primates got back a separating bone secondarily. The other branches are of no interest for us now, but Figure 1 shows the general scheme.
Now, it can be seen that the synapsid/anapsid and synapsid/diapsid divergences are much older than the "reptile/mammal" one, so differences are large and conveniently clear between mammals and recent reptiles; but this implies that such differences can only by chance be used to distinguish mammals from their own ancestor reptiles. Indeed, many distinctive "reptile/mammal" criteria are rather diapsid/sinapsid ones. Henceforth we restrict ourselves to synapsids, reptilian or mammalian. The synapsid systematics can be found in the synapsid monography of KEMP (1982) which we will use extensively in this paper. However we will depart from it in one point of nomenclature. We will call the entire branch Synapsida, to oppose them to the other branches. We can do this, because each member is synapsid for skull. Synapsids are very probably monophyletic.
The lower grade of Synapsida is Pelycosauria; there were never doubts that they were all reptiles. The upper grade is Therapsida; there are some argumentation whether therapsids were mono- or diphyletic. Kemp's opinion is that they were monophyletic and we may accept this because we are not interested here in the pelycosaur/therapsid border.
But the Therapsida/Mammalia grade border is in question. The border is obscure, and the very nature of the grade Mammalia depends on the definition. However the name of the upper grade refers to mammae, whose presence is awkward on fossils, nonexistent on duckbills, and not necessarily correlates with the actual definition. So we will use the term Mammalia, or mammal, but in quotation marks, referring that the name must not take in face value. We omit the quotation marks for recent mammals, even if monotremes have no mammae. So the grade "Mammalia" is opposed with the lower grade Reptilia, which latter is acidentally monophyleticB; or, more directly, with the (sub)grade Therapsida. Then the main question of this paper can be formulated as follows.
Did the last common ancestor of all recent mammals belong to the grade Therapsida or to the grade "Mammalia", according to some reasonable, unambiguous and operative definition? If it was therapsid, mammals are polyphyletic and clade Mammalia does not exist in a natural systematics. If it was "mammalian", then mammals are monophyletic and then the term mammal can be used both as a grade and as a natural group in taxonomy of recent animals; then the true mammalian characteristics are of common origin.
Let us try to answer the question by using "common sense". Assume that we are able to draw at least two lines instead of a single "Therapsid/Mammal" border. There is a lower border below which therapsids were "obviously non-mammal", and an upper border above which everybody was "clearly mammal"C. There remains a grey zone where we cannot clearly distinguish, but never mind.
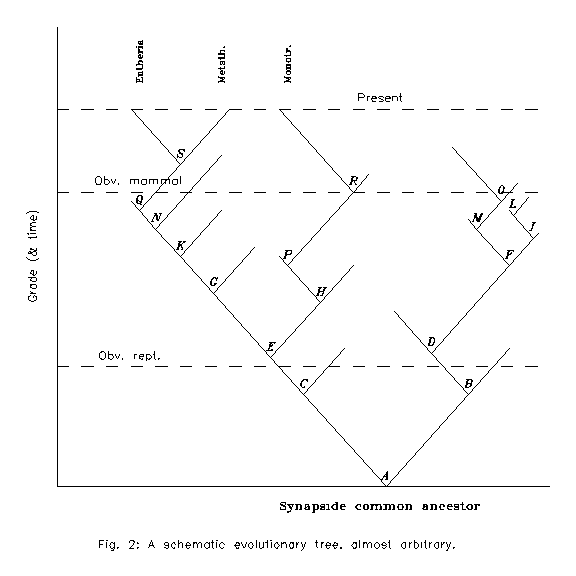
Assume that the scheme is Figure 2 (it is handmade and the only realistic points are one common synapsid ancestor down and 3 recent mammal groups at the top) and the demarcation lines run as drawn. Then all recent mammals are monophyletic because the common ancestor E is above the reptilian zone, but fossil mammals are not all monophyletic with the recent ones because the common ancestor of all the mammals, recent or fossil is A, a reptile. But to decide via such a scheme one would need exact demarcation lines and exact descent lines: the first is slightly subjective, the second is unknown for monotremes. So even this way is not operative.
|
|
Figure 2: A schematic evolutionary tree, almost arbitrary.
Figure 115 of KEMP (1982), showing therapsid evolution, ends somewhere in Lower Jurassic, and the author mentions the fossil hiatus between basal and Middle Jurassic for therapsids. Three closely related branches reach the hiatus: the "mammals” (meaning morganucodontids and Kuehneotherium), the tritheledonts and the tritylodonts; all other therapsids seem extinct already in basal Jurassic. As we shall later see, the general evolutionary levels of these three branches are rather similar at the Tr/J boundary. For example the secondary D/Sq joint is present then in "mammals", in the tritheledont Diarthrognathus, and possibly in Tritylodon maximus.
When the continuous synapsid fossils reappear in Middle Jurassic, tritylodonts reappear with Stereognathus and probably die out soon. The other therapsid descendants are primitive "mammals", monophyletic or not. The obvious and usual explanation is that tritheledonts died out during the hiatus.
However in the present state of art "mammal" and tritheledontid descendants cannot be told apart, not having valid distinctive features between the two groups. Kemp himself states that it would be easy to unite "mammals" and tritheledonts, and the new taxon would be monophyletic.
The above arguments suggest that there are serious problems in deciding between mono- and polyphylecy of mammals. Still, we would like to compare the evolutions on the lines leading to therians and monotremes. The general direction was almost the same on both patways but definitely faster on the first. The relation of the two pathways is that of a highway and a sidetrack.
SOME CLASSIFICATIONS OF MAMMALS
Mammalian classification started from recent mammals. The terms vary, and we will not mention all systems.
According to general opinion recent mammals are classified with two forkings. They are either therians or non-therians. The Theria are either "placentals" or "marsupials". But the presence of a placenta is very probably a grade, i.e. the placenta may be polyphyletic. Here we mention only one argument. The marsupial Australian rat in family Peramelidae does possess some kind of a placenta (GÉCZY 1989), therefore it developed the placenta independently. Appendix A gives some considerations about the evolutionary gain of the placenta; henceforth we use the more neutral terms Eutheria and Metatheria. KEMP (1982) unites them in the group Ditremata. In contrast to therians or ditremes, there are the monotremes, atherians, non-therians, or Prototheria, roughly equivalent terms for recent animals. (However, Atheria are defined via non-therian molar teeth, and monotremes via the single excretion opening, not necessarily correlated.) Maybe Ditremata/Monotremata is the older forking, but even at this point there may be some doubt. We will return to this point in Appendix B, here we mention only one item.
Eutherians, metatherians and monotremes form a logically triangular structure. Eutherians and metatherians are similar in the therian structure of molars, but not in the jaw and ear structure. Eutherians and monotremes are similar in the jaw and ear structure but not in the structure of molars. Metatherians and monotremes are similar in the original number of incisors, in the presence of epipubis, and in a number of plesiomorhic structures. (In details see Appendix B.)
More specifically, the therapsid articulare and quadratum have become the middle ear auditory ossicles incus and malleus in all groups. The third lower jawbone, the angulare, goes to tympanic bone in eutherians. Now this structural rearrangement is the same in monotremes (MURRAY 1984), while in metatherians there is a strange angular process below the hindpart of the dentale and the tympanon is fixed by an independent bone, not by the tympanicşangulare. On the other hand, recent therian molars all went through a phase in which the molar cuspules were arranged in triangular fashion, while monotreme cuspules are, and very probably, were not. Even if we accepted that the metatherian-monotreme similarities all were plesiomorphic, the forkings among recent mammals may be more complicated as generally and optimistically believed.
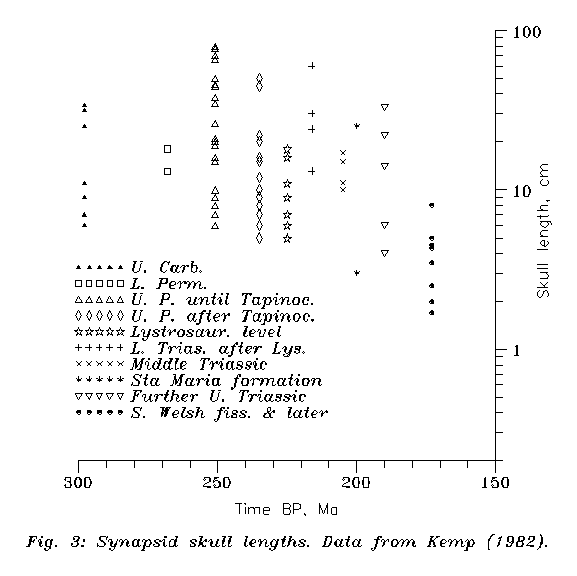
Now let us try to include Jurassic, Cretaceous and Tertiary fossils. The therian-nontherian distinction is based on dental structure, mainly on that of molars, and not accidentally so, because from Jurassic and Cretaceous layers most "mammalian" fossils are only teeth. This is so because synapsid skulls underwent serious shrinking from Upper Triassic (see Figure 3), so early "mammals" were mouse-sized and most of their bodies were too fragile. Now the present therian molar structure can be traced back, through patterns ancestral to it, to the Upper Triassic Kuehneotherium (KEMP 1982) (or, as we shall see, on equal right to the tritheledontid Pachygenelus?). They may or may not be called therian, but the subsequent Jurassic symmetrodonts and Jurassic and Early Cretaceous eupantotheres are clearly therians. Early Cretacean Aegialodon may have been near to the common ancestor of all recent therians for tooth structure and functioning. If this structure is relevant, then the eutherian/metatherian split is not earlier than cca. 130 Ma, and the monotreme/therian split is older than that (see this later). The non-therian monotremes then must have evolved independently.
|
|
Figure 3: Synapsid skull sizes vs. time. Redigitalised for all skull drawings of KEMP (1982) for which scale factors have been given. The epochs abbreviated on the Figure are: 1: Upper Carbo-niferous; 2: Lower Permian; 3: Early Upper Permian including Tapinocephalus deposit; 4: Upper Permian after Tapinocephalus deposit; 5: Lystrosaurus level of Lower Triassic; 6: Further Lower Triassic; 7: Middle Triassic excluding Santa Maria deposit; 8: Sta. Maria deposit (Middle/Upper Triassic border); 9: Further Upper Triassic; 10: South Welsh fissures (basal Jurassic), later Jurassic & Cretaceous
Several non-therian branches existed, being that a negative definition. "Non-therian" means a not therian (i.e. not trigonal) cusp pattern. But such a pattern can either be something before the trigonal pattern, or something evolved into a different pattern, see some details in Appendix B. To non-therians belong the first accepted "mammal", the Upper Triassic Eozostrodonş Morganucodon, the very poorly known Rhaetic haramiyids, Jurassic triconodonts and docodonts, and Jurassic, Cretaceous and Lower Tertiary multituberculates. The latest group persisted until the end of Eocene, but there is even a suspected offshot in the Miocene, Desmostylus, to which we return later.
Unfortunately, recent monotremes are rather poor in teeth. The spiky anteaters ("echidnae") are completely toothless, and the duckbill ("platypus", a misname, having an insect priority in the name) has only juvenile teeth, some not even erupting. However, fossil platypoid teeth are known from the 1970's, as far back as the Early Cretaceous Steropodon galmani (cca. 110 Ma, PASCUAL et al. 1992, ARCHER et al. 1992, AUGEE, 1984). The cuspule arrangement of these teeth are vaguely similar to multituberculates or maybe to docodonts, but not to symmetrodonts or eupantotheres. (The matter is still obscure. PASCUAL et al. (1992) are rather critical about the therian character of ornythorhynchid molars, while ARCHER et al. (1992) regard it as pre-tribosphenic therians. KIELAN-JAWOROWSKA & al. (1987a) also permits that they be non-tribosphenic therians branched from the mainstream between Peramus and Aegiolodon. Some more years of analysis may settle this question, but in almost all taxonomic systems monotremes are non-therian.)
Let us add one more fact. D/Sq articulation is known from Upper Triassic in Eozostrodon, Diarthrognathus, and, at least as opinion of some authors, Tritylodon maximus, or even in Middle Triassic (Chańares) Probainognathus (KEMP 1982). (For the liquid nature of the taxonomy, the first one is "mammal", the other three are therapsids, respectively a tritheledont, a tritylodont and a chiniquodont). But as for the loss of the original Art/Q articulation, we can tell as follows. Kuehneotherium had a through on the medial side of the mandible very probably still housing the articulare joined to quadratum, and other postdentary bones. Now the drylestid eupantothere Crusafontia (probably Upper Jurassic) still has a similar through (see Figure 105 of KEMP 1982), so possibly had still the postdentaries. Then for articulation (and for auditory apparatus!) Crusafontia is only as "mammal" as Eozostrodon, although it is already therian.
Unfortunately, while until the uppermost Triassic the therapsid data are almost continuous and numerous, in the Jurassic and Early Cretaceous therapsid and "mammal" fossils are rather exceptional, especially so in Lower Jurassic. Therefore the interpolation is not unambigous. Up to the seventies the general idea was as follows. The therian mammal ancestors are obviously the eupantotheres and symmetrodonts; one needs a ancestral form to symmetrodonts and then can interpolate through it to an appropriate Upper Triassic therapsid, possibly to Diarthrognathus, the ideal transition form. Then the ancestor of the only recent non-therians, the monotremes, must be somewhere among docodonts, triconodonts or multituberculates, most probably the last one, being it the most persistent, and further back the lineage goes either to Diarthrognathus, or to another therapsid (see e.g. GÉCZY 1979). In the second case mammals are diphyletic.
For the multituberculate-monotreme link Desmostylus was a candidate, a strange-looking Miocene mammal, whose molars were similar to the multituberculate, haramiyid and maybe monotreme molars: the cusps are arranged into two parallel rows. see e.g. the separate Desmostylus japonicus molar in the museum of the Geological Survey of Japan, Tsukuba (GUIDE BOOKLET 1992), where the two parallel rows of three cylinders are clear.
An alternative opinion, contrary to the molar structure, was that Desmostylus would be transitional between Sirenia and Proboscidea, both therian.
GREGORY (1947) derived monotremes from marsupials, by neoteny. AUGEE (1984) calls him the "last serious exponent of this idea"; however KEMP (1982) sympathizes with therian origin of monotremes, which, doubtless, would simplify the evolutionary tree.
As one can see, views are various and different that, by citing the appropriate author, one can argue either the mono- or the polyphyletic origin of recent mammals. Here we mention one more problem. Dentition pattern is believed almost as important in phylogenetic reconstruction as the structure of individual teeth. “Most primitive” patterns are reconstructed. Of course, terms as "most primitive...pattern" possess clear-cut meanings only if there is some "Time's Arrow" or "irreversibility" principle involved. About mammal dentology there is such a principle. Let us cite e.g. PASCUAL & al. (1999) who say: "...among Mammalia...the addition of supernumerary molars is extremely rare". Maybe diphyodonty sets the temporal horizon of such a notion, and it is put cca. at Eozostrodon. (CROMPTON & LUO (1993) claims that all early Jurassic "mammals", excepting Sinoconodon, were diphyodont.)
Now, the reconstruable most primitive eutherian pattern is
3 1 4 3
‑‑‑‑‑‑‑
3 1 4 3
but the metatherians (marsupials) have a quite different primitive pattern
5 1 3 4
‑‑‑‑‑‑‑
5 1 3 4
Now, the 5 incisors cannot be derived from any known "mammal" dentition pattern; even in Eozostrodon the number of incisors is 4. So for individual cusp pattern of teeth eutherians and metatherians are near to each other and seem to have diverged only in the Cretaceous, while for the dentition pattern metatherians seem to go back independently to the therapsid stage. As for monotremes, complete toothy jaws are available only for the juveniles of recent Ornithorhynchus, where the pattern is the rather unfamiliar
0 1 2 3
‑‑‑‑‑‑‑‑‑
5 1/2 2 3
Note that, as will be seen, 2 canines occur only at therapsids. Appendix B gives some dentition patterns for comparison.
So the picture is still unclear. In the next section we try to approach the problem from the other end and very briefly recapitulate the synapsid (mainly therapsid) evolution.
THE FUNDAMENTALS OF SYNAPSID EVOLUTION
Here we follow mainly the monograph of KEMP (1982) about "mammal-like reptiles". But first, for purposes which will be clear only below, we try to give absolute ages instead of qualitative terms. This is easy if we do not require too high accuracy. Namely for the main borderlines we take the results of Holmes and Kulp (FARQUHAR, 1967) and average them; then interpolate for layers according to Figure 1 of KEMP (1982). As the two most important borderlines, P/T is then at 227.5 Ma and T/J at 180.5 My. In more details Figure 3 can be used for epoch-time conversion of the present paper. (While the exact numbers here can be criticised, the relative chronology will be solid until we use solely the averages of Holmes & Kulp.)
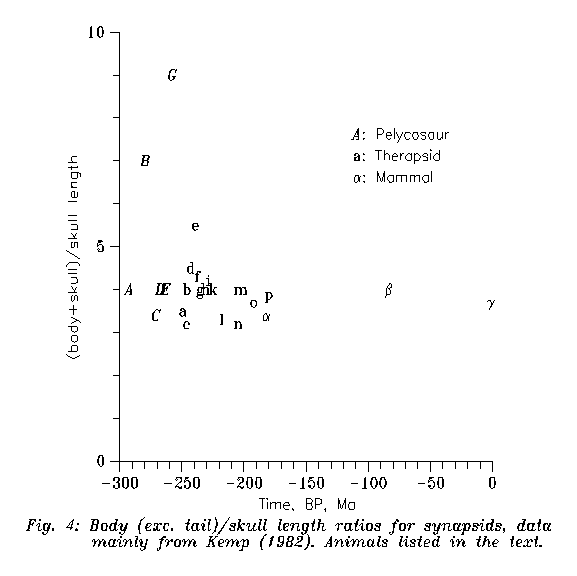
Now, synapsids had sometimes surprisingly homogeneous trends. First let us see a quantitative example. Figure 4 gives the ratios of full body lengths (without tail, practically to the end of the pelvic) and skull lengths. We measured all figures of KEMP (1982) where the full skeleton was reconstructed; Pleistocene Diprotodon is there for Quaternary comparison. It is interesting that the ratio, with minor exceptions, was about four during the last 300 Ma (!) and no divergent evolution is seen among synapsids in this characteristics; if anything, convergence is seen. (By the way, the relative constancy of this ratio, quite foreign in the brach Diapsida, is lucky for us: for first approximation mere skulls are sufficient to estimate body sizes.)
|
|
Figure 4: Body/skull lengths for synapsids. All drawings of KEMP (1982) are redigitalized if the complete skeleton + scale factor have been given, appended with Pleistocene Diprotodon from GÉCZY (1979). Labels: Pelycosaurs: A Archaeothyris, B Edaphosaurus, C Varanosaurus, D Dimetrodon, E Varanops, F Mycterosaurus, G Cotylorhinchus. Therapsids: a Titanophoneus, b Galechirus, c Robertia, d Moschops, e Cistechephalus, f Procynosuchus, g Lycaenops, h Dicynodon, i Thrinaxodon, k Ericiolacerta, l Kannemeyeria, m Massetognathus, n Probelosodon, o Exaeretodon, p Oligokyphus. "Mammals": a Megazostrodon, b Zalambdalestes, g Diprotodon
Similarly locomotion evolved from complete sprawling gait to that of the forelimbs but more and more erect gait (vertical proximal limb) in the hindlimbs, and further. Monotremes are now at the advanced but not final therapsid level. Dentition in each group evolved from simple, homodont and conical dentition to a differentiated one, and at the most advanced therapsids teeth clearly separate into incisors, canine(s), premolars and molars. In the lower jaw there is a constant tendency for the dentale to extend more and more backward, gradually atrophising the postdentary bones. Thermal control is also evolving into the same direction in synapsid groups. We hardly know anything about postnatal care and milk secretion. However milk glands are modified sweat glands (more specially, modifications of the more ancient apocrine variant having role also in emanating sexually attractive odours), so, in principle, excretion of some nutritive fluids were possible after the development of sweat glands, which must have gone hand to hand with thermal control and hair.
The jaw hinge needs a somewhat more detailed discussion. With the continuously growing dentale it was more or less natural that the back end of the lower jaw was approaching the squamosum. With this continuous shift in advanced cynodonts, maybe first in Cynognathus, the surangulare, just above the angulare, reached the squamosum, and they formed a secondary articulation. Maybe this connection existed also in Diademodon, but via ligaments (KEMP 1982). Afterwards, for a while, the advanced cynodonts had double articulation, Art/Q and Sa/Sq. This second connection stabilized the lower jaw against being dislodged if the prey moved too violently.
THE ENVIRONMENTAL CONDITIONS DURING THERAPSID EVOLUTION
So far the picture has not become coherent. Let us look now at the environment in which therapsids were evolving towards the mammal stage. The reason for it is that some trends may have been driven by the terrestrial environment. It is pointless to refer to the environment in the jaw articulation; as seen, that was an inevitable consequence of the continuous expansion of the dentale, and, no doubt, all therapsid groups would have reached at least the double articulation sooner or later, had they survived. However thermal control, viviparity, postnatal care and nutrition &c. seem to be answers to a harsher and harsher environment. The problem is that there seems to be a phase inconsistency.
It would be easy to explain the correlated chain of events via the Permian glacial period (GÉCZY 1996). In a very cold environment thermal control is an advantage. Poikilotherm reptiles would have continuous problems in cold climate and they would have transient problems in moderate climate in the morning. In additions, the young, with the higher surface to volume ratio would have even more serious problems. So it would be better to retain the embryo until a more mature phase, to keep them in a nest, to take care of them and to feed them. Indeed, there was a persistent low temperature stage of the Permian from the end of the Lower Permian to the Permian/Triassic boundary (BUDYKO et al. 1987), although the glaciation is not reflected too well in the global temperature; it needed too a periantarctic position of Gondwana as well. No doubt, thermal control had been developing during that period, sometimes hair is reported and the body sizes increase, which helps in the surface to volume ratio. The therapsid radiation also occurs during this time of cold. However the rapid shift toward "mammalness" happened afterwards, during a warming up. What is even stranger, the advance is then correlated with the diminution of therapsids, as if in a warming climate the therapsids themselves were creating their own problems in keeping themselves warm to drive the evolution. This is, of course, impossible.
One may try with 3 explanations. It is possible that therapsids were forced to avoid conflicts with the emerging advanced diapsids, by size reduction; however the Triassic diapsids were no match for the therapsids. It is possible that the big therapsids had the danger of overheating in the warming situation and they solved the problem not by better thermal control but by increasing the surface/volume ratio. However it is better to consider temperature, oxygen and carbon dioxide data together. It seems as if, by the sequence of events, the earlier therapsid strategy almost carried them into a cul-de-sac, putting great challenges on them. Namely, on the C/P boundary the oxygen level was cca. 1 PAL and CO2 level was cca. 10 PAL. The Permian thermal minimum started with 0.7 PAL in oxygen, and CO2 was also down with 4 PAL. Then along with the subsequent warming the oxygen level continued to decrease, with a minimum of cca. 0.4 PAL in the middle of the Triassic. (For more details see e.g. BÉRCZI & LUKÁCS, 1998.) So it was harder and harder to maintain high metabolic level.
Now, O2 support becomes easier at small sizes. True, lungs are not exactly surfaces, but fractal objects with a dimensionality 2<n<3; still that means that O2 support becomes better with a size reduction. On the other hand, in the Middle Triassic temperature maximum the global terrestrial temperature was cca. 23 C°, so the heat loss of small animals does not seem to have been serious. From the uppermost Triassic both "mammals" and surviving tritylodontids were mouse-sized animals. Advancing diapsids did not have this problem, because they were poikilothermic so did not need high metabolic rates. The 1 PAL oxygen level was again reached in the Middle Jurassic, no surprise that "mammal" fossils became then more abundant. The maximal oxygen level occurred at the end of Lower Cretaceous with 2.7 PAL, but the temperature was high enough. The 1 PAL was again reached in the Palaeogene. Anyway, Upper Permian and the P/T boundary were rather hard to life (ERWIN 1993). Superanoxia lasted in the seas some 10 Ma (ISOZAKI 1994), although here we are not too interested in aquatic life.
With utmost caution one may try to guess when did the sweat glands appear. One possibility is the Lower Triassic, when the glaciation passed, but the animals were still substantially large so they may have met with internal overheating. The other possibility is the Jurassing warming with increasing oxygen level. The animals then could have made profit from higher metabolism but they had to get rid off the heat excess. But in both cases lactation may be polyphyletic. If sweat glands are from Lower Triassic, then there was time enough to mutate into milk glands even before the divergence of the common final line into tritylodonts, tritheledonts and "mammals"; if they are Jurassic, their mutations into milk glands may be late enough to be independent on diverging "mammal" branches.
Finally we note that some authors suggest the idea that the Permian was ended by a cosmic influence. Somewhere at Upper Permian or P/T boundary there was indeed an external influence. However analogies with the K/T boundary seem misleading. The K/T extinction seems to have been caused by a chondrite body of several km diameter (small asteroid or maybe cometary core). But the P/T boundary lacks so simple an explanation. We can quote the opinion of a palaeontologist as "...Asteroid impact was hardly a cause for the series of catastrophes at the Permian-Triassic boundary." (GÉCZY 1996). On the other hand, in some Japanese and Chinese layers MIONO (1998, and citations therein) verified the correlation between spherule density and appearance of mudstone, considered a signal of mass death (ISHIDA & al. 1992). Now, spherules are considered as necessary signals of impacts, but in the present case their compositions (mainly FeO) are hardly compatible with impact. MIONO (1996) rather suspects a galactic cloud. Sometimes, and especially in aquatic layers, the P/T boundary is almost sterile (see e.g. ISHIDA et al. 1992), and extraterrestrial spherules in sediments are more and more frequent as we approach the P/T boundary (TÓTH et al. 1997). However, the P/T boundary was not catastrophic for therapsids, and the critical time for the „reptile-mammal” transition was not the P/T boundary but rather mid-Triassic.
MAMMAL CRITERIA
While, of course, it is possible to define Mammalia in such a way that it be monophyletic, the resulted taxon might then be formal. E.g. the two extremes are as follows. We may define mammals as eutherians, but then obviously warm-blooded suckers are left out. On the other hand we may define mammal via having prismatic enamel on the teeth (KEMP 1982), and then all suspected mammals + tritheledontids together are monophyletic mammals. The problem is not the inclusion of the not too well known tritheledontids but that prismatic enamel has nothing common with the general idea of mammals.
So here we try with something else. Let us see if and when animals arrived at the mammalian grade. We first define a group which is doubtless mammal. Because we try to locate Monotremata among synapsids, we first ignore them in this definition, and there remain the therian synapsids as the core of mammals. It defines a grade, not a clade. Then we try to determine if monotremes have acquired the given property, and that when it was acquired first in the branch Synapsida. Where the answer is not simply Y/N or a date, a numbered note will be done afterwards. 0 stands for characteristics utterly impossible to date, so not numbered. The first column names the (eu)therian stage, in the second the Y/N states if living monotremes share the therian character. The third column mentions the first known occurrence of the property. Absolute times will be given BP, in Ma, according to the timescale of this paper. Shorthand references are as follows:
K KEMP 1982
PE AUGEE, 1992
AC ARCHER & CLAYTON, 1984
PH PARKER & HASWELL, 1962
G GÉCZY 1989
WR WALTON & RICHARDSON, 1989
Then we get Table 1. We admit that the properties listed below are of no homogeneous importance. Still comparing many such properties is some help. Sequential numbers are for reference in Figure 5, when at least estimated ages are available.
|
Therian Property |
Monotremata |
First synapsid occurrence |
|
Teeth |
|
|
|
Differentiation to: |
|
|
|
1 precan., canines & postcanines |
Y |
Dinocephalians, 268, K |
|
2 incis., canines, & postcanines |
Y |
Dvinia, 233, K |
|
3 premol. vs. mol. |
Y, PH |
Cynognathus, 216, K |
|
Occlusion by: |
|
|
|
4 Incisors |
? |
Dinocephalians, 268, K |
|
5 Molars |
Y |
Diademodon or Cynognathus, 216, K |
|
6 Canines1 |
? |
Pachygenelus, 1822,3, K |
|
7 Cusps on molar in triangular (or descended) pattern |
N |
Pachygenelus, 1822,3,4, K Kuehneotherium, slightly later?, K |
|
8 Diphyodonty |
Y? |
Eozostrodon, 182?, K |
|
9 Prismatic enamel |
Y |
Tritheledonts, 182; Eozostrodon, 182, K |
|
Lower jaw hinge |
|
|
|
10 Secondary, to squamosum too |
Y |
Diademodon, 216, K |
|
11 Secondary, Sq/D too |
Y |
Probainognathus5, 205 |
|
12 Primary, "reptile" Ar/Q lost |
Y |
Symmetrodonts, cca. 150? Eupantotheres after 150?6, K |
|
Lower jaw |
|
|
|
13 Angulare leaves jaw7 |
Y |
Upper Cretaceous? 110 |
|
14 1 bone |
Y |
Multituberculates, 150?8 |
|
15 Triangular motion at mastication |
N |
Morganuconodontid and Kuehneotherid common ancestor, >182, K |
|
Skull |
|
|
|
16 Postorbital bar vanishes |
Y |
Tritylodontids & tritheledontids, cca. 190, K |
|
17 Prefrontal vanishes |
Y |
Tritylodontids, cca. 190, K |
|
18 Side wall with large alisphenoid |
N, AC9 |
Cynodonts from 233? K |
|
Ear |
|
|
|
19 Malleus & incus |
Y |
Eupantotheres after 150?6 |
|
20 Tympanic from angulare10 |
Y |
Primitive Eutheria? Cretaceous?11 |
|
Neck |
|
|
|
21 7 cervic vert.12 |
Y, HP |
Procynosuchus, 236, K |
|
22 Cervic ribs absent |
N |
110?13, 14 |
|
23 Atlas-axis fused |
Y |
150?15 |
|
Rump |
|
|
|
24 Costal plates absent on ribs |
Y |
Exaeretodon, 192, K |
|
Limbs & girdles |
|
|
|
25 No independent coracoid |
N |
110?16, 17 K |
|
26 No interclavicle |
N |
150?16 |
|
27 Humerus roughly vertical |
N |
Oligokyphus18, 182 K19 |
|
28 Femur roughly vertical |
Y |
Diademodon, Cynognathus, 227, K |
|
29 Digital formula 2.3.3.3.3 |
Y |
Therocephalians, 246, K |
|
30 Trochanter minor |
Y |
Oligokyphus and Eozostrodon, 182, K |
|
Soft parts |
|
|
|
31 Diaphragm |
Y |
Procynosuchus, 236, K |
|
32 Hair |
Y |
Cynodonts, 227?20 |
|
33 Double circulation |
Y |
Thrinaxodon21, 227, K |
|
Thermoregulation |
|
|
|
34 Endothermy |
Y |
Cynodonts, 233 |
|
0 Constant body temperature DTŁ4 C° |
Y |
? |
|
0 Constant body temperature DTŁ1 C° |
N |
? |
|
Excretion |
|
|
|
35 Hypertonic urine |
Y |
?, after Diademodon, <216, K |
|
36 2 excretion openings |
N |
Eu- and metatherian common ancestor? 135? |
|
Reproduction |
|
|
|
0 Lygaeus type sex determination |
Y22 |
? |
|
0 One pair of sex chromosomes |
N |
? |
|
0 Viviparity |
N |
? |
|
0 Uterine luteal phase |
Y, AC |
?23 |
|
37 Milk secretion |
Y |
After Diademodon24, <216, K |
|
0 Mammae |
N |
? |
|
38 Maternal care |
Y |
? Cynodonts? AC |
|
Brain |
|
|
|
39 Neocortex |
Y |
Probainognathus, 205 (QUIROGA, 1980) |
ľľľľľľľľľľľľľľľľľ
1: The therian way: lower with the antero-laminal of the upper.
2: Tritheledontid.
3: Not in synchronous Eozostrodon (K), a "mammal".
4: Slightly.
5: Probably a chiniquodont.
6: The drylestid eupantothere Crusafontia still seems to have the through housing the complex rod
of postdentaries in earlier forms, so Art still was probably attached.
7: Not in Metatheria (G).
8: Now in Eutheria and Monotremata. No trace of postdentaries or angulare has been found in
Multituberculates.
9: Alisphenoid is generally small in non-therian "mammals", but not in morganucodonts.
10: Not in the therian Metatheria?!
11: Also in monotremes. Multituberculata?
12: Vary in some eutherian (?) Xenartha.
13: Only in therians?
14: Strongly reduced but present in Oligokyphus (tritheledont) and Megazostrodon ("mammal") at
and slightly after -182 Ma. Absent in Late Cretaceous Zalambdalestes.
15: Not in morganucodontids (K).
16: Still present, albeit reduced, in Eozostrodon (K).
17: Present but reduced in multituberculates and in Early Cretaceous Gobinocodon ostromi
(ARCHER et al. in PE).
18: Tritylodontid.
19: Transient stage in Eozostrodon (K).
20: Foramina for vibrissae are detected (K).
21: Indirect evidence.
22: With variation, involving several X and Y chromosomes (Bick in EP).
23: It may or may not have existed in any cynodont through shell of non-cleidotic eggs.
24: Even the smallest found Diademodon individual had functional teeth, so it may have fed itself
independently from birth. On the other hand, milk glands are modified sweat glands, so
they are in principle possible after hair & endothermy.
Table 1. Therian vs. "reptile" characters, and the first appearance of the character
|
|
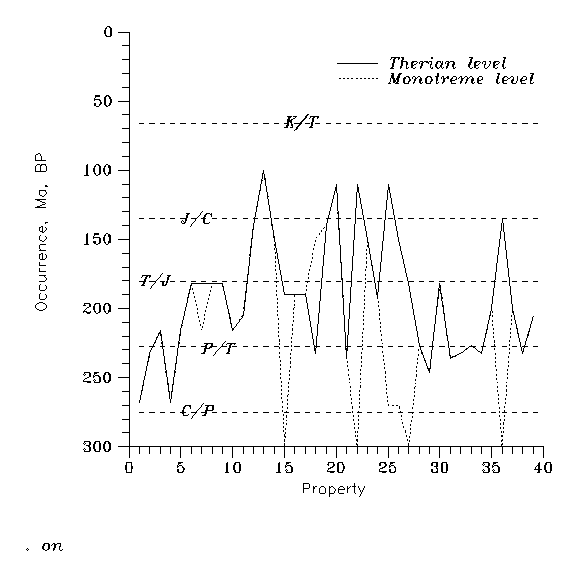
Figure 5: Temporal appearance of "mammal characteristics", on monotreme and therian level. Numbers refer to Table 1.
The above 46 criteria (of which 39 are dated at least approximately) are all distinctive between recent Theria/Reptiles. However very probably 21 (more than half of the dated) already had appeared in some therapsids before the "first mammal" (for definiteness before Eozostrodon), more 3 appeared synchronously with the "first mammal", but not in it, and further 2 appeared simultaneously in the "first mammal", and in a "non-mammal" therapsid. Therefore more than 2/3 of the above distinctive features are useless in "mammal synapsid vs. non-mammal therapsid" discrimination. In addition, monotremes do not satisfy a quarter of the above "mammal vs. reptile" (in fact therian vs. diapside) criteria.
Now let us make a Gedankenexperiment, based on the fact that "terminal therapsids" were generally small, so their postcranial skeletons are often fragmentary or lost. Let us construct a "synthetic advanced cynodont" with all the progressive (i.e. therian) features of non-mammal (in the sense of Kemp's clads) cynodont branches appearing until the "first mammal". Now this "synthetic cynodont" would (and so might) have possessed 27 of the 39 datable "distinctive" therian features. Which is surprising (but for exact numbers an accident), is that this number is just the same for morganucodonts ("first mammals") in that time, and for present monotremes. So on semiquantitative level present therian mammals are equally distant from a very advanced synthetic non-mammal therapsid (existed or not), from the "first mammals" and from present monotremes.
A slightly more quantitative result (still not necessarily to be taken in face falue) can be constructed as follows. Let us select the not extremely correlated characteristics. Four of the 46 are very correlated with neighbouring ones, so now we neglect 1, 11 and 13 of the numbered ones, plus the looser one of the temperature control; the remaining 42 are taken as independent directions in a 42-dimensional Euclidean space. Put therians (all Y) into the origin, any other group at coordinates 0 if Y, and 1 if N, while stochastically to 0 and 1 with probabilities 1/2,1/2 if the answer is still ?. (This construction may get some credit by referring to the many convergences and parallellisms in the tritylodont-tritheledont-early "mammal" complex (LUO 1994).) Then we get the following distances (T=Theria for present therians, M for monotremes, E for Eozostrodon and S for the "synthetic progressive therapsid”):
|
|
|
sTM |
= |
3.61 |
± |
0.07 |
|
|
|
sTE |
= |
4.06 |
± |
0.22 |
|
|
|
sTS |
= |
4.00 |
± |
0.25 |
|
|
|
sME |
= |
3.39 |
± |
0.33 |
|
|
|
sMS |
= |
3.67 |
± |
0.30 |
|
|
|
SES |
= |
2.92 |
± |
0.30 |
Then all recent mammals are roughly at equal distance from each other, from Eozostrodon, or from the "synthetic therapsid", no difference is significant at 2s level. The Eozostrodon-therapsid distance is smaller but they both evolved less. The Theria-Monotremata distance is slightly smaller than average, but the present distances have been formed from phenotypes, so common mammal selection may be behind. (This is either a numerical confirmation of LUO (1994)'s statement about convergences, or a signal that the schemes are improper, or both.) However these data do not rule out even a possibility that monotremes are connected with "early mammals", while therians to therapsids!
This semiquantitative measurement indicates that "terminal therapsids" and "first mammals" were rather similar, in the Upper Triassic Kemp's "mammal" lineage did not differ qualitatively from the other advanced cynodonts, and that the "distictive therian/reptile features" are almost useless to distinguish them in the uppermost Triassic. The monotremes must have had either very peculiar evolution or a divergence point earlier than -182 Ma. Indeeed, the two dozen Ma hiatus between basal and Middle Jurassic (KEMP 1982) is at a very important location.
Recently KIELAN-JAWOROWSKA (1997) emphasized the importance of multituberculate lineage in understanding early mammalian phylogeny. In this context it is worthwhile to note that multituberculates were the dominant mammals in Cretaceous, which lasted longer than Tertiary. Our Table 1, however, do not contain an extra column for multitiberculates, because in this paper we are interested about the relation between monotremes and therians. Still the inclusion of multituberculates may change logical structures. We mention only one example.
Our Property 9 is prismatic enamel. That is acquired at Uppermost Triassic/Basal Jurassic by a "mammal" and a tritheledont. So far so good.
However KEMP (1982) states more: that it is a "mammal"/tritheledont synapomorphy. (He says that it is "...one derived character of mammals possessed by tritheledontids...On the strength of this fact, it may be concluded tentatively that the tritheledontids are phylogenetically most closely related to the mammals".
This seems indeed true if multituberculates are excluded from the "mammalian" lineage (see his Figure 112, where multituberculates, together with haramiyids, stand totally apart, no ancestors and no descendants, truely Allotheria). But if multituberculates are also "mammals", then prismatic enamel is surely no synapomorphy. Tritheledonts acquired it in Uppermost Triassic (KEMP 1982), but some multituberculates during Jurassic did not yet do. Paulchoffatia did not yet have prismatic enamel and similar was the situation for most other Plagiaulacoidea (PASCUAL & al. 1999; KIELAN-JAWOROWSKA & al. 1987b). It seems as if multituberculates had acquired this property in Early Cretaceous, and then independently either of Eozostrodon or of tritheledontids.
GENETIC DISTANCES AMONG MONOTREMES AND METATHERIANS
WESTERMAN and EDWARDS (1992) made a large step forward when they performed a DNA hybridization measurement series to get genetic distances among 3 monotremes (all the recent genera as well as species) and 3 metatherians. Without going into details, it is based on the effects of continuous mutation (say, by cosmic radiation) on the DNA double helix. It is changing on some places, and then H bridges become impossible between the mutated-unmutated points (PIMENTEL & MCLELLAN 1960, BICZÓ et al. 1966). Therefore the dissociation temperature between the DNA of different species goes down, roughly linearly with the time of independent evolution. Observe that now environmental effects are almost negligible. (Most changes are neutral, not appearing in phenotype, so selection has only a rather minor role.) The method was described in details in WESTERMAN & EDWARDS (1991) and we do not question it. In the 1992 measurement 6 species were used as follows:
|
Oana: Ornythorhynchus anatinus, monotreme from Australia |
|
Tacu: Tachyglossus aculeatus, monotreme from Australia |
|
Zbru: Zaglossus bruijni, monotreme from Australia |
|
Dros: Dasykaluta rosamondae, metatherian from Australia |
|
Meug: Macropus eugenii, metatherian from Australia |
|
Dvir: Didelphis virginiana, metatherian from America. |
Besides the distances, the measurement can even check Gregory's hypothesis, because if the 3 monotremes as a compact group are located among metatherians, then they originate from a particular metatherian group, and are not opposed to Metatheria as a unit.
The result was quite interesting. By the optimal tree (least error squares) they got a first split between the only American species and the 5 Australian ones (either metatherian or monotreme) followed very soon by an Australian metatherian/monotreme split. Assuming strict constancy of the mutation rate, and by setting the time scale via putting the australo/amerodelphid split (for whatever reason; see CLEMENS et al. 1989) to 128 Ma, the time interval between the two splits would seem only 8-9 Ma, with an undiscussed margin of error, and the time of both splits are very close to the J/K boundary and so to the eutherian/metatherian split. Let us recapitulate: according to the Westerman-Edwards measurements the evolutionary scheme turns out to be as follows. Maybe first happened an eu/metatherian split, then among the metaterians a geographic split, finally a marsupalian/monotreme split a la Gregory in Australia.
Now, this result is very important, and deserves a proper statistical analysis, in a way which automatically yields the statistical errors too. This will be done just here, by means of a simpler and therefore more transparent statistical method. The idea is as follows.
We have a tree (postulated at the beginning) with a few binary forkings. Then we fit one time (or average distance) to each forking, via least squares (JÁNOSSY, 1965) from all lineages starting from the same forking. We do know that this evaluation has one flaw: on some paths descendants fork into recent measurable animals only after some common evolution, so some parts of the mutations are not independent. However this is not a serious problem if the measurement errors are substantial: then double counting of two, very lately asundered species means simply doubling the number of measurements for almost the same species, and the 1/n1/2 rule will hold for the measurement of the same species as well as for the independent mutations after split. Corrections could be made by iterative determination of the independent lives of species, but now it seems unnecessary.
Westerman and Edwards measured distances (temperature differences) in both directions (i.e. "hot" Oana - "cold "Tacu" and "hot" Tacu - "cold" "Oana") and tried to perform several measurements for all configurations, to get the statistical errors. (Distances must be the same in both directions.) For Zbru, because of the shortage of the material, this was not always possible. Hence it is possible to reconstruct all the distances and errors, which are the inputs of our analysis. The steps to this input are described in Appendix C; here we give the results in a tabular form as Table 2, where everything is in C°; above the diagonal we see the weighted averages of the distances, below the statistical errors of the averages.
|
|
Oana |
Tacu |
Zbru |
Dros |
Meug |
Dvir |
|
Oana |
* |
13.56 |
14.61 |
24.26 |
24.78 |
25.92 |
|
Tacu |
0.69 |
* |
0.04 |
22.84 |
23.70 |
23.73 |
|
Zbru |
1.07 |
0.06 |
* |
19.74 |
24.07 |
24.78 |
|
Dros |
0.86 |
0.70 |
0.26 |
* |
18.86 |
23.11 |
|
Meug |
0.64 |
0.72 |
1.92 |
0.34 |
* |
22.53 |
|
Dvir |
0.98 |
2.26 |
2.90 |
0.09 |
0.19 |
* |
Table 2. DNA substitution distances (upper right) and standard uncertainties (lower left) in °C between monotreme and marsupial species. Details in Appendix C.
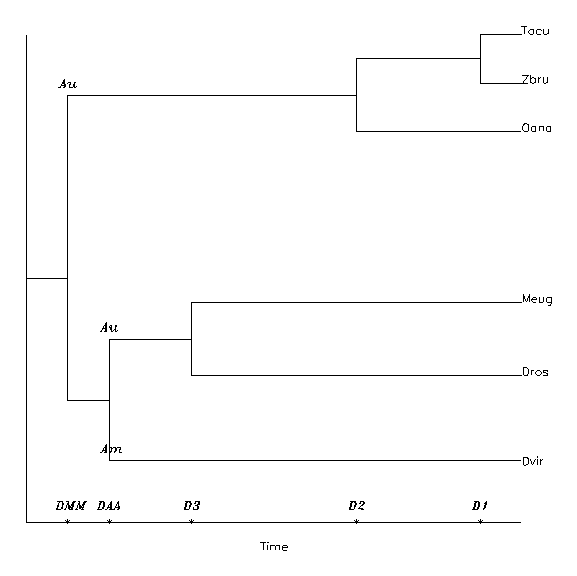
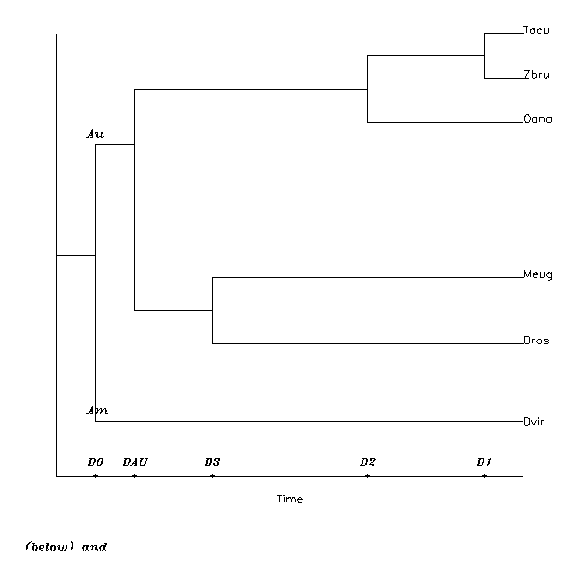
Now let us see two trees of different structures. Scheme WE is the tree structure which emerged from the analysis of WESTERMAN and EDWARDS (1992), with 5 splits. D1 is the distance equivalent of time from present to the Tacu/Zbru split, D2 is that to the last common ancestor to all recent monotremes, and D3 is to the last common ancestor of Dros and Meug. DAU is the distance to the Australian metatheria/Australian monotreme split, and finally D0 is the first forking in their analysis, the Australian/American one. In the scheme this forking represents the amerodelphid/australodelphid split too, which will be fixed to 128 My, so defining the temperature-time conversion.
The other scheme is the classical phylogenetic one. Down to D3 it is the same as above; then back in past comes the amerodelphid/australodelphid split DAA, and the first one, DMM, is the fundamental one, Metatheria/Monotremata, without any reference to the Meta-/Eutheria split, before or after. The two schemes can be visualized on Figure 6.
|
|
|
|
Figure 6: The "taxonomic" (above) and "Gregory" (below) split schemes for evaluating DNA distances. The D's are the distances of present and the split events. D1: Tachyglossus aculeatus/Zaglossus bruijni split; D2: last common ancestor of all recent monotremes; D3: last common ancestor of Dasykaluta rosamondae and Macropus eugenii, both Australian metatherians. Especially for the Westerman-Edwards ("Gregorian") scheme: DAU: the Australian metatheria/monotreme split; Do: the last common ancestor of the 6 species. Especially for the taxonomic scheme: DMM: the monotreme/metatheria split; DAA: the australodelhid/amerodelphid split.
As told above, all these distances are calculated from weighted averages of the distances leading to the respective split, and the weights are, as usual, the inverse squares of errors given in the Table.
Then taking all data in face values,
D1 = 0.04 ± 0.06
D2 = 13.87 ± 0.58
D3 = 18.86 ± 0.34
DAU = 22.65 ± 0.21 DAA = 23.00 ± 0.08
D0 = 23.03 ± 0.08 DMM = 21.45 ± 0.21
Obviously, the classical phylogenetic scheme does not seem compatible with the data (DMM < DAA), while the "Gregory-type" scheme in which Australian metatherians are closer to Australian monotremes than to American metatheres is viable. (Observe that such a result is incompatible even with the idea that monotremes are therians. Even the KIELAN-JAWOROWSKA (1987a) scheme would put the monotreme branching away well before the Meta/Eutherian split.)
However now comes the chi˛-probe. Since the weights are derived from the uncertainties of the distances, if a split is real, different distances must fall near to the same D value and the measure is just the statistical error of the particular distance. By other words, let us form the expectation value of a chi˛-type quantity
Chi2 = i=1n (Di - D)2/(dDi)2 (7.1)
from the individual distances belonging to the same split, where Di's are the individual distances between a fixed split and present on different lines and D is the weighted average for the distance of the split. Chi2 will be a stochastic variable with cca. chi˛ distribution and with
<Chi2> = n-1 (7.2)
(JÁNOSSY, 1965).
It is enough to perform the test for the oldest two splits; afterward the schemes are identical. Now, in the first scheme
|
|
Chi2AU = 142.60, |
n-1 = 5 |
|
|
Chi20 = 16.87, |
n-1 = 4 |
|
|
Chi2AA = 7.61, |
n-1 = 1 |
|
|
Chi2MM = 119.72, |
n-1 = 8 |
The chi2 tests clearly rule out both sets of results.
Further investigation shows that the largest inconsistency occurs in both schemes at the split among whose descendants there is the Zbru-Dros distance. Table 2 shows that the particular distance is too short with a too small error. The discrepancy can also be formulated in terms of the triangle inequality.
D's are some distances in some virtual space, so let us check them by means of the triangle inequality (EISENHARDT, 1950). In any Riemannian space of positive signatures in a triangle the sum of length of any 2 sides must be larger than the length of the third side. Now, consider the (Tacu, Zbru, Dros) triangle. In it
DDrosZbru+DZbruTacu = 19.78 ± 0.27; DTacuDros = 22.84 ± 0.70
so the triangle inequality does not hold, above 4s.
Remember that there was a shortage in Zbru material, so the Zbru-Dros distance was measured in both directions only once. For any reason the only measurements seem to have fallen somewhat short (see the triangle inequality), and in the same time near each other. Such a fluctuation is possible from only 2 measurements. At least the measurement error is not reliable, and the proper weights are unknown. Then DZbruDros with its present error (impossible according to the chi˛ test) distorts the results. The minimal arbitrariness is to omit this single date. After the omission we get
D1 = 0.04 ± 0.06
D2 = 13.87 ± 0.58
D3 = 18.86 ± 0.34
DAU = 23.95 ± 0.35 DAA = 23.00 ± 0.08
D0 = 23.03 ± 0.08 DMM = 24.15 ± 0.33
Now the "Gregory-type" scheme is not self-consistent, while the classical phylogenetic one is such. Observe that the two schemes give similar results to similar questions. DAU and DMM are not too different, and they both represent monotreme/metatheria splits; and D0 and DAA are both ameridelphid/australodelphid splits with practically the same values. But the reemergence of a "Gregory-type" pattern was an artifact of an anomalous DZbruDros distance signalled by both the chi˛ test and the violation of the triangle inequality. By removing this single highly suspicious distance the usual phylogeny returns; as far as for the metatherian-monotreme relation. Still it is interesting that Gregory had some good reasons to construct his neothenic theory: there is still a slight tendency to get shorter distance between a monotreme and an Australian metatherian than between a monotreme and an American one. However now the tendency is not significant:
<DMonotr-Au.Metath.> = 23.92 ± 0.35
<DMonotr-Am.Metath.> = 25.50 ± 0.86
These data seem to suggest something. But for the difference
DMonotr-Au.Metath. - DMonotr-Am.Metath. = 1.58 ± 0.93
which is well below 2s level and therefore generally considered as not significant indeed.
Fixing now DAA to the suggested 128 Ma, the reanalysed results of the Westerman-Edwards measurement can be summed as:
|
Monotreme-Metatherian split: |
134.4 ± 1.8 Ma |
|
Amero/australodelphid split: |
128.0 ± 0.4 Ma |
|
Macropus/Dasykaluta ancestor: |
105.0 ± 1.9 Ma |
|
Ornithorh./tachyglossid split: |
77.2 ± 3.2 Ma |
|
Tachyglossus/Zaglossus split: |
0.2 ± 0.3 Ma |
Of course, the above errors are valid only if the mutation rate is indeed such that that particular time is 128 Ma. Still, accept it with some caution. Then the monotreme-Metatheria split was just at the Jurassic/Cretaceous border, disturbingly near to the suspected Eu/Metatheria split set generally to cca. 135 Ma (CLEMENS et al. 1989). Again the impossible triple forking is haunting the problem. This shows that the pattern is a complex one; it may become clearer if eutherians will be incorporated into the distance measurements.
This measurement is superior to anything using phenotypes; if the measurements are repeated several times and if the molecular clock is regular on all lineages.
Anyway, then monotremes have an existence old enough to find Steropodon galmani in Lower Cretaceous (ARCHER et al. 1985) with a definitely monotreme and non-therian molar. Then monotremes are not much younger than multituberculates (if at all), and if they were living fossils (i.e. if their mutation rate were slower than normal), then their existence could go back even to Middle Jurassic, to triconodonts, docodonts and other non-therians. In any other points we can remain still as baffled as before this analysis. Namely, e.g., Oligokyphus had a non-sprawling gait some 50 Ma earlier than the independent monotreme existence, still sprawling now.
CONCLUSION 1: HIGHWAYS VS. SIDETRACKS
The main goal of the present paper is not to decide the mono/polyphylecy of mammals, but to show a well-documented example for evolutionary pathways in highway/sidetrack relationship with each other. Such a relationship is a qualitative, not a quantitative matter; in some cases it is not clear if the relationship is such or it is simply some more or less parallel evolution. However it seems that the monotreme/therian case is such. In the pathway(s) from the first primitive synapsids to present mammals there seem to have been 3 regimes: i) divergences among pelycosaurs; ii) strong common trends and parallel evolution among therapsids; and iii) a "highway vs. sidetrack" relation between the ways to us and to monotremes. The paths leading to extinct "mammal" groups could have been anything; Jurassic fossils are rare and we do not know the soft bodies in the necessary extent to decide.
We do not deal with the pelycosaur grade in any details. If one looks at Figure 4, he sees divergent tendencies there in one aspect.
About therapsids we list some examples for strong common tendencies and parallellisms; for further details cf. KEMP (1982), especially his Chap. 12. The examples are as follows:
1) Body/skull lengths. Figure 4 demonstrates that among therapsids (and especially after 233 Ma BP) no serious divergences appear in this ratio; the animals are hardly distinguishable in it even from present mammals.
2) Teeth differentation. There is a common advancing precanine/canine/postcanine, later incisor/canine/molar differentation in various therapsid branches. In all the latest therapsid groups the extent of differentiation was on or near to "first mammal" level, although the special tooth patterns may have been different.
3) Secondary jaw hinge. There was a common therapsid tendency for the backward expansion of dentale, maybe for the advantages of a solid lower jaw (sutures are lines of weakness). During this process it is not surprising at all if some lower jaw bone reached the squamosum, and then the lower jaw became stabler against being dislodged by the fighting prey, a fatal case. In our days the primary/secondary articulation is a valid distinctive mammal/reptile feature, but there "reptile" means "diapside reptile", where this trend did not work.
At 216 Ma BP the squamosum was reached by the surangulare in Diademodon, a diademodontid advanced cynodont, but the expansion trend did not stop, and some 11 Ma later the growing articular process of the dentary invaded the new secondary hinge and so formed the "mammalian" articulation in the advanced chiniquodontid cynodont Probainognathus in South America. Again, in no more than two dozen Ma later the D/Sq articulation appeared again, in not less than 3 branches: Tritylodon maximus (probably), the tritheledontid Diarthrognathus, and the "mammal" Eozostrodon, possibly independently. It seems that the "mammal" secondary articulation was only a matter of time; every advanced cynodont branch got it if did not die out before.
4) Locomotion. It seems that the general trend was a more and more sagittal proximal limb bone, faster on the hindlimb than on the fore one. The possible driving force was greater speed; the sprawling gait of recent Tachyglossus is energetically more economic than the therian way (EDMEADES & BAUDINETTE 1975), and may fit better to rough terrains (KEMP 1982), but it is obviously slower. If predator and prey are both therapsids, a common tendency can be understood. The "therian" "erect" stage of femur seems to have developed independently among tritylodonts and therian mammals (and we do not know the tritheledont stage).
5) Differentiation to pectorial and thoracic regions. This is important for the existence of diaphragm, hence for higher metabolism &c, therefore investigated in details. In the early stage all ribs were similar albeit longest in the "mid-pectorial" region. Then in Procynosuchus a sudden qualitative change appeared between the 20th ("last pectorial") and 21th ("first thoracic") ribs. The difference becomes greater and greater as time goes by. In stem cynodont (?) Thrinaxodon and trasvertodont Massetognathus the thoracic vertebraes are already as ribfree as in mammals while in later tritylodontid Oligokyphus thoracic ribs exist but are very vestigial (KEMP 1982). Therefore these stages had been reached independentlyD.
It seems that in the therapsid stage the evolutionary rates did not differ too much on different lineages, for which a quantitative example is the D/Sq articulation reached at least 4 times in 23 Ma. So it would be hard to distinguish highways from sidetracks; maybe simply parallel pathways are seen. In contrast, in the therian/monotreme relation the monotreme forelimb action is still at a level passed by "final therapsids" and "first mammals" 182 Ma ago.
Now let us turn to the third, "mammal" stage, and let us see first if there were really more than one pathways. Namely, it is also possible that monotremes simply lag behind us on the same pathway, so they are archaic and nothing else. We investigate 3 aspects.
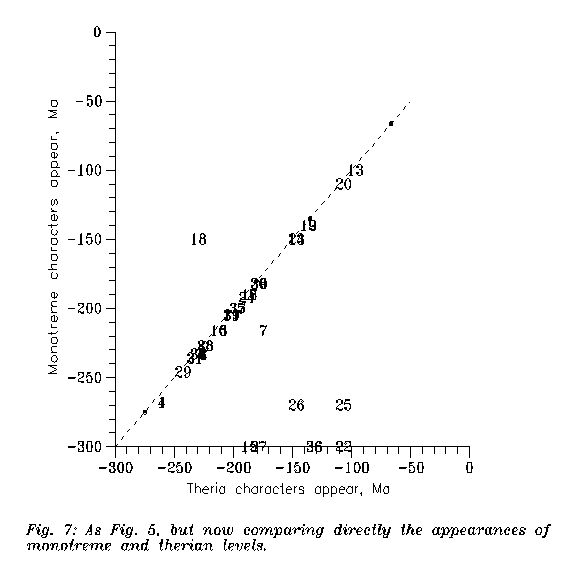
The first is Figure 7, from the same database as earlier Figure 5: when did therapsids reach the monotreme and the therian level? If monotremes were simply archaic, then all points would lay in the lower right half. It is generally so, but not for feature 18, the lateral wall of the braincase; the therian structure seems to be a cynodont heritage while the monotreme-multituberculate structure seems an innovation absent in therians. And note also that monotreme lower jaws and tympanics are "more eutherian" than the metatherian ones.
|
|
Figure 7: As Figure 5, but now comparing directly the appearances of monotreme and therian levels. Numbers refer Table 1, epoch borders are heavy dots.
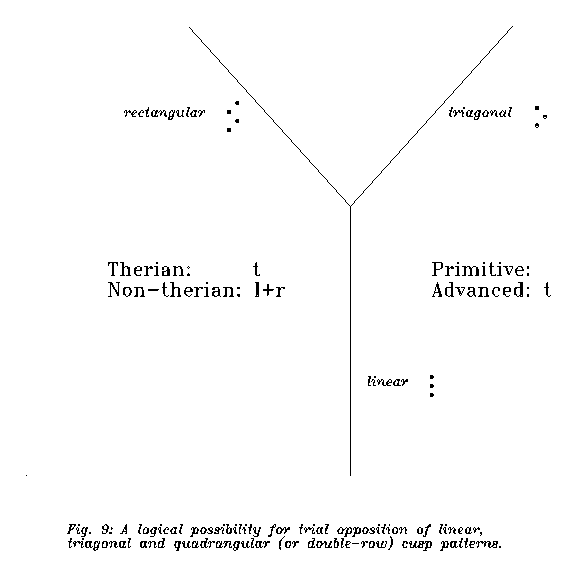
The second aspect is the molar cusp structure. The original opposition was lineal/trigonal, and since the old cynodont way was lineal, the trigonal structure may have been an innovation while the lineal one archaism. But in more advanced "non-therian" mammals the structure is not lineal. In docodonts and multituberculates an alternative solution was found to the trigonid one: some rectangular pattern. In multituberculates (and, interestingly, in Desmostylus, anything it may have been) there are two rows of cutting edges and at occlusion one ridge fits into one walley. We leave the discussion of therian/atherian nature of Ornithorhynchus, Obdurodon, Steropodon and Monotrematum molars to experts, but they doubtless do not fit into a classic Cope-Osborn scheme. So the terms "atherian" for molars covers two patterns: one archaic and one alternative to the therian pattern. Monotreme molars are not simply plesiomorphic compared to ours.
The third feature is tricky. Monotremes seem more advanced as us in the general degree of ossification. Archaic reptilians were less bony and more cartilaginous than later ones and mammals; but monotreme skulls are more ossified than ours and the sutures are almost unidentifiable in mature ones. The same phenomenon seems to appear in the sclera, where a cartilaginous structure replaces the fibred connective tissue (GRIFFITHS 1989): a higher degree of "ossification". However, this latter feature may be plesiomorphic as well, because the structure is present in recent Sauropsida (ÁBRAHÁM 1964). Now diapsid reptiles+birds were not ancestral to mammals. The feature is plesiomorphic only if synapsid reptiles have the homologous cartilagous structure, and this feature does not fossilize.
Then the monotreme pathway is different from the therian (or tribosphenic therian, if monotremes turn out to be pre-tribosphenic therians (ARCHER et al. 1992; KIELAN-JAWOROWSKA & al. 1987a)) one. But the pathways diverged surprisingly moderately. For this see Figure 7: the monotremes are either on therian level or plesiomorphic to therian in the most "reptile/therian distinctive features". The two pathways are almost parallel, but evolution was much slower on the monotreme one.
CONCLUSION 2: ORIGINS
Now it is proper to return to origins: of the monotremes and of ours. But first let us see if the question has a clear meaning at all.
Monotremes seem monophyletic. As shown above, the monotreme line is independent from cca. the J/C boundary and Steropodon galmani, with ornithorhynchid tooth, is Lower Cretaceous. All fossils upwards have such teeth, and nothing is known about tachyglossid teeth. Since the tachyglossids separated in the uppermost Cretaceous (WESTERMAN & EDWARDS 1992; also this paper), nothing indicates diphyletic origin.
However, this is not so with us, eutherians. Eutherians are defined by an alternative: existence of placenta, and trigonal, tribosphenic (or derived) molars. But the problem is that the monophylecy of the placenta is not proven. There is a counterexample (the Australian marsupial rat), four different structures of the placenta and the presence of a strong evolutionary pressure to develop it (see Appendix A).
The molar cusp pattern is believed to be more reliable; and based on it the therian lineage is guessed to start with Kuehneotherium, or if not, from symmetrodonts; then pantotheres may have been a symmetrodont offshot, and therians a pantothere offshot (GÉCZY 1979, SEVERCOV 1981, KEMP 1982; and many others not cited here). But there are "therians" with placenta but without triagonal or tribosphenic molar cusps. Only as examples we list a few.
Zalambdodonts are "archaic insectivores with molars of special structure"; the tanrec on Madagascar still has a cloaca (GÉCZY 1979).
Xenarthrans have special vertebrae articulation, and the teeth are very primitive (or advanced?). Some of them have 100 teeth in one jaw. Either the therian teeth decayed into many parts, or they never followed the therian pattern; for any case nothing indicates an earlier therian tooth pattern, since the dentition is such from first appearance in the Lower Eocene. Exceptionally among mammals, here the number of neck vertebrae can be anything between 6 and 9. In addition the metabolic rate and locomotion is slow.
Pholidotids are archaic with an almost complete scale covering, glands restricted to mouth and perianal regions as in reptiles, primitive columnar stapes (as in monotremes and some marsupials) and exceptional number of neck vertebrae. They are toothless, and then no therian molar pattern can be seen.
Tubulidentates have no incisors or canines and what they have in the place of molars, does not follow the "therian pattern"; some thousand narrow dentine cylinders are present, with no enamel at all. Ancestors before miocene are unknown, so there is no evidence for therian teeth.
Desmostylians had vaguely multituberculate-type molars built up from 6-8 independently enameled cylinders but in two rows.
Some recent cetaceans are toothless, some others have peculiar dentitions. Some dolphins have more than 200 homodont conical teeth, while Eocene Protocetus had conical (so canine- or pelycosaurian-type) incisors.
While some of these "erratic therians" may have had originally therian molars, there are too many such types and sometimes the "specialised" dentition is accompanied by very archaic other features; so some independent lineages may be misclassified as eutherians. We cannot and would not done anything with them here, but to demonstrate in what extent these uncertainties disturb even the present discussion, we mention Sudamerica ameghinoi from Patagonian Banco Negro Inferior (very early Tertiary) layers which was first classified as a xenartran (SCILLATO-YANÉ & PASCUAL 1985), but later as a specialised multituberculate (KRAUSE & BONAPARTE 1990), while now PASCUAL & al. (1999) state that the question of the position of Sudamerica among mammal clades has reopened. If a non-therian may be classified to eutherian or vice versa then anything may happen among mammals.
Henceforth we ignore the doubtful groups of "eutherians" and focus on "therians proper". Now let us see the ideas for therian and monotreme origins. They changed very much in the last quarter century.
Until the first successes of monotreme archaeology (WOODBURNE & TEDFORD 1975, ARCHER, PLANE & PLEDGE 1978) the origin of monotremes was highly speculative. The general idea was that as a non-therian, it may be connected to docodonts or multituberculates. The juvenile Ornithorhynchus molars may remind somebody to multituberculate molars. From 1972 morganucodontids were considered too; or it may have come from any advanced therapsid. Let us see some variants without pursuing completeness.
One idea was multituberculate origin via Desmostylus. While Desmostylus itself may come from a very persistent multituberculate (or may not), monotremes now cannot be derived from Desmostylus. The latter is from Miocene, and the independent monotreme branch is justified from Cretaceous by fossils.
KERMACK and KIELAN-JAWOROWSKA (1971) believed that multituberculates and docodonts had originated from a common quasimammal ancestor, and monotremes had been an insectivorous sister-taxon of herbivorous multituberculates as back as Upper Triassic or Lower Jurassic; later in the Jurassic monotremes were outcompeted by eupantotheres everywhere outside Australia. If so, therians and non-therians go back to two, different, non-mammalian, therapsids.
In 1979 GÉCZY interpolated as follows. There are four lineages to the mammalian grade. Multituberculates, leading perhaps to monotremes, originate from the tritylodont Bienotherium. From the Ictidosaurs (now tritheledonts) near to Diarthrognathus started the triconodonts, docodonts root in bauriomorphs, and the origin of the symmetrodont line, ending in therians, is uncertain.
From the end of 70's adult monotreme teeth are available, the molar pattern is known in greater details. The similarity to multituberculate molars seems slightly awkward ("Their morphologies give almost no clue about their relationships; AUGEE 1984)); they may even be pre-tribosphenic, i.e. early diverging, special therians (ARCHER et al. 1992) as well as non-therians (PASCUAL et al. 1992).
GÉCZY (1989) starts with the Upper Triassic Morganucodon (now Eozostrodon), and thence, through divergences, originate all the non-therians, and maybe the therians.
SEVERCOV (1981) clearly assumes a monophyletic origin from Morganucodon (either mammal or not) with independently diverging branches to monotremes, triconodonts, multituberculates, symmetrodonts, pantotheres and docodonts, while the line to therians starts in Upper Jurassic from the neighbourhood of pantotheres; the therian radiation is in the Upper Cretaceous.
KEMP (1982) distinguishes two "mammalian" lineages. One starts from an unknown ancestor, and then forks into haramiyids and multituberculates, ending with the latter. The other starts from a very advanced therapsid, from the neighbourhood of tritheledonts and/or tritylodonts. The first divergence goes to triconodonts, docodonts and morganucodontids, the second one to symmetrodonts and kuehnotherids, the third to eupantotheres, the fourth maybe to monotremes, the fifth to aegilodontids, and then finally the main lineage ends in the therians.
AUGEE (1984) suggests a tentative evolutionary tree as follows. Mammals are either mono-, or polyphyletic. In the first case there was an early forking into therians and non-therians; the latter ones then forked into two pairs of sister-taxa, one is multituberculates/monotremes, the other is triconodonts/docodonts. However, if mammals are polyphyletic, then monotremes are relict therapsids which gained milk gland, endothermy and hair in the therapsid stage, while the D/Sq articulation and the shift of the articulare and quadratum into the middle ear happened parallelly with the therians.
MURRAY (1984) does not take sides in mono/polyphylecy, however emphasizes his doubts about the strengths of some arguments for the monophyletic origin. About the D/Sq link, identical in therians and monotremes, notes that "...the dentary-squamosal articulations may have been attained by more than one therapsid group through parallel evolution" (we have seen earlier that it was indeed so) and so it "...could link together a number of groups that might not have shared certain other skeletal advances...". He even seems to identify a small prefrontal bone, in which case monotremes would have diverged earlier from therians that the tritylodontids did (KEMP 1982). Also he notes that post-natal care evolved long before vivipary, and it may have been important in evolution of social interactions "...bringing to mind packs of co-operatively hunting cynodonts and doting synapsid mothers nuzzling fuzzy young in their nest". The general picture (suggested or not) seems to be that monotremes may have originated from almost any advanced carnivore cynodont, separate or not from the therian ancestral therapsid. For a parallel we quote here PARKER & HASWELL (1962): [monotremes and triconodonts] "...are extremely archaic mammals that have diverged from the main stock (if they do not represent a separate stem altogether) perhaps even before any of the Upper Jurassic mammals..." (then morganucodontids were not yet known) and the presence of par excellence mammal features in them "...leads to speculation concerning the possible degree to which synapsid reptiles may have progressed towards lactation and homoeothermy...", and to speculation that "...the monotremes represent an independent 'warm-blooded' line, derived by Triassic cynodonts...". Such ideas have not been ruled out by the existence of Upper Triassic Eozostrodon, or by fossil monotremes; they are made improbable only by the WESTERMAN & EDWARDS (1992) distance measurement making an obstacle and therefore the necessity of at least a check to anybody preferring independent monotreme line before the Jurassic.
We close this listing with a number of observations giving fragmentary insights into the problem of origin. LILLEGRAVEN & KRUSAT (1991) from the investigation of the Late Jurassic Haldanodon exspectatus, a docodont, conclude that docodonts are basal mammals, and they claim that "parallel development of similar features was an all-pervasive phenomenon within the early evolution of the Mammalia.", a statement very important when investigating highway/sidetrack relations. KREBS (1991) investigated an eupantothere, Henkelotherium guimarotae, and found reduced coracoid, so the monotremes with non-reduced coracoid "...must have separated from the line leading to Theria before the Middle Jurassic - probably even having completely independent origins.". Similarly, Early Cretaceous Gobinocodon ostromi (JENKINS & SCHAFF 1988) and multituberculates (KRAUSE & JENKINS 1983) had reduced coracoids, while in monotremes the coracoid is unchanged. These data all would prefer a pre-mammalian divergence of monotremes; but there are again the Westerman-Edwards measurements preferring a Late Jurassic or Early Cretaceous divergence (except if monotremes and metatherians diverged together very early, but metatherians seem therians).
Then we remain with no clear way out. Omitting all fine details, compare nothing other than shoulder girdles and DNA distances. Monotreme forelimb action is more primitive than that of the tritylodontid Oligokyphus, and the monotreme shoulder girdle is more therapsid that anything else "mammal". This indicates a divergence from the therian line earlier than the tritylodont-therian split. Then the common ancestor was very probable a therapsid reptile, not mammal. But DNA distance measurements put the split to the J/C boundary, 50 Ma later. On the other hand, the coracoideum was already reduced in a therian, the eupantothere Henkelotherium, before the J/K border and monotremes, diverging later, do not show this reduction. Something is basically wrong.
The simplest explanation would be a slower mutation rate. Then the cca. 24 °C difference in dissociation temperature of pure and fused DNA double helices would have been produced by more than 135 Ma. But then all timetables are confused, and ameridelphid-australodelphid asundering would go to the end-Triassic, which is highly improbable. An alternative would be lower mutation rate in the past, but that is improbable too. The mutation rate depends on two factors: cosmic (and terrestrial) radiation flux and repair mechanisms of cells. We see no reason, why and how the galactic cosmic radiation flux could have been lower in terrestrial Mesozoic, and the terrestrial concentration of radionuclides is decreasing, not increasing. The solar magnetic field somehow shields Earth, but there is no indication for a different solar mode of operation in the Mesosoic (the Permian glaciation may have been caused by a transient solar convection instability on the border of the core, as well as the Later Tertiary cooling and finally glaciation, maybe correlated with the solar neutrino problem (FOWLER 1972)). And in earlier times cellular repair mechanisms are expected to have been less advanced.
There is a desperate possibility which we only mention. There is a complicated meiotic chain of chromosomes in monotremes, including sex chromosomes (BICK 1992) but maybe some others too. This chain may be the first step in collecting all sexually important features together, but it seriously restricts the recombination of parental chromosomes (see Appendix D). Is it then possible that the lack of recombinations make the genetic clock slower, and then the DNA distances should be recalculated for times? We think that this mechanism does not influence too much. Point mutations have the same rates. Phenotypic changes may slow down, but we do not see effective mechanisms to slow down neutral mutations. The problems have remained.
We close the conclusion with an amusing possibility. Kemp's concluding evolutionary tree (Figure 115 of KEMP 1982) reconstructs the final therapsid, tritheledont-mammal forking in later Middle Triassic; tritheledonts die out just after the Tr/J boundary and mammals persist.
However we do not have to conclude to tritheledont extinction. True, no tritheledontids are identified any more. But observe that tritylodonts persisted until Middle Jurassic, so the environment was tolerable for therapsids. Now observe that we have no distinctive feature between mammals and tritheledonts at hand. Both have prismatic enamel, which may be connected with longer lifetime of teeth, therefore diphyodonty and so possibly even lactation (KEMP 1982). (To this point we note that FRASER ROBERTS (1967) mentions a sex-coupled gene whose defect results in non-prismatic enamel. It would be tempting to think that the wild form represented the original cynodont stage and the mutation happened in the common ancestor of tritheledonts and "mammals". However the present human manifestation is such that in the hemizygote males there is no enamel at all while the non-prismatic enamel appears only in heterozygotic females. But this gene cannot be the original "enamel" gene of synapsids either because in homozygotic cases the enamel is automatically prismatic. This point would deserve further analysis.) Secondary jaw articulation is present at at least one tritheledont.
It seems, anyways, that convergences were very frequent during early tritylodont/tritheledont/mammal evolution (LUO 1994). No surprise that we cannot distinguish between "mammal" and tritheledont offshot too when fossils reappear in Middle Jurassic. It is not impossible that some Middle Jurassic animals descended from tritheledonts. (But maybe not the multituberculates; primitive ones, namely Paulchoffatia, and haramiyids, had non-prismatic enamel, as tritylodonts had, see PASCUAL & al. 1999.)
But if this is possible, then it is not impossible either that early therians came from the tritheledonts? We have seen that the molar cusp pattern of the tritheledont Pachygenelus was slightly triangular while that of Eozostrodon was not. And then we cannot disprove even the possibility that archaic atherians including mammaeless monotremes come from "mammals", while we, therians, may not be mammals in cladistic sense, but very advanced tritheledonts.
We agree with ARCHER et al. (1992) in that "...the question of monotreme origins remains central to our understanding of mammalian evolution and is as yet unanswered". But, with the rate of the present research, it may not be unanswered for long.
ACKNOWLEDGEMENTS
The authors would like to thank Drs L. Hably, G. Koppány L. Kordos, I. Molnár and A. Pahl for illuminating discussions. Partly supported by OTKA T/026660.
APPENDIX A: ON THE PROFITS OF DIFFERENT MAMMALIAN WAYS OF REPRODUCTION
It seems that the three different ways of mammalian reproduction form a gradual scale. If so, and if there was a serious evolutionary pressure, then placentals may be polyphyletic.
Let us see the gradual scale. Monotremes lay eggs and hatch them; but the eggs are not cleidotic and takes nutritive fluids in the "uterus". So there is a possibility that the egg becomes overborn and breaks up in the uterus. Then instead of the egg an immature embryo would be born.
Exactly this happens in marsupials: in Didelphis the egg is broken on the 10th day of the 13 day long gestation in utero (GRIFFITHS 1989). The newborn marsupial needs an almost sealed off environment for a while. Obviously it would be better for the child to retain the embryo within the mother. On the other hand, bioomechanically it would be better to the mother too, because in the uterus the excess weight is almost at the center of gravity, while in a pouch gravity acts on it creating a persistent torque, which disturbs the balance and motion.
However the growing embryo cannot be kept inside without a filtering mechanism, because it is as different from the mother in immunologic sense as a sister (50% of identical genes). Transplantation between sisters is impossible without very serious treatments, so incompatibility endangers the mother and of course the child too. There is then an alternative: either premature birth, or a filtering mechanism closing off the macromolecules with immunologic effects (similarly to the non-cleidotic egg shell?).
In Homo sapiens gravidity starts with an interval when mechanical problems are still absent but there is a general bad feeling, stomach problems &c. Then the placenta develops cca. at the end of the 3rd month, and the general bad feeling vanishes but mechanical problems increase. In this time the human embryo can make only very limited movements, so this stage is a rough analogon of the just hatched monotreme or the newborn marsupial. Cca. after the 7th month the immunologic problems restart, but then the embryo is already more or less mature and can leave the mother if needs be. The obvious explanation is that the embryo is already too large and the filtering activity of the placenta becomes insufficientE.
So the placenta is a good solution if a marsupial would need longer incubation, mutually good for mother and child. Therefore for marsupials the need is persistent for developing placenta; the constant need may have led to repeated independent appearances of placenta in non-placental mammals.
APPENDIX B: THE (MONOTREME, METATHERIA, EUTHERIA) TRIANGLE AND THE DENTITION PATTERNS
We have mentioned the problem in the (monotreme, metatherian, eutherian) triangular relation of splitting. Let us now see this more explicitly.
The three groups of living mammals form a triangle from the viewpoint of (supposedly) very important and conservative characteristics. Let us choose three of them, all so important that they were used for classification: molar pattern, dentition pattern and lower jaw & ear bony structure. The first one is used to define therians vs. non-therians, the second one was used to trace back large groups among eutherians, and the third is a popular "mammal/reptile" demarcation line. For brevity we use O as Ornithorhynchus for Monotremata (Tachyglossus dentition is unknown), M for Metatheria and E for Eutheria.
M and E oppose O, because the old molar cusp pattern of them was triangular, while O followed a different pattern back to Lower Cretaceous (ARCHER et al., 1995).
M and O oppose E because they have 5 original incisors (recent O in the lower jaw as juvenile teeth, PARKER & HASWELL, 1962; known fossils have not retained incisors) while there is no evidence for 5 incisors in any known ancestor of E's; the reconstructed primitive E dentition has only 4 in both jaws (GÉCZY 1989, KEMP 1982).
O and E oppose M because the angulare shifted from their lower jaws to the ear region and became the tympanic; in contrast in M the tympanum is fixed by a bone of different origin (GÉCZY 1989) and the angulare either has completely been lost or now forms the excessive angular process (ancestral Australian Metatheria are poorly known). And note that the evolution of ear ossicles was important; it determined the improvement of auditory sense. But here is again some surprising point. LUO, CROMPTON & LUCAS (1995) emphasize the importance of a "mammal" apomorphy: the petrosal promontorium. By it the cochlea was housed by a single bone, not three as in nonmammal cynodonts, and maybe the new organisation was superior for acoustics. Let us accept this idea. Then again let us quote KEMP (1982), about the tritheledont Diarthrognathus: "The fenestra ovalis lies entirely within the periotic bone as in mammals and in contrast to all other cynodonts, where it is partly surrounded by the basisphenoid and the basioccipital bones". Again, as if a mammal character appeared also in tritheledonts.
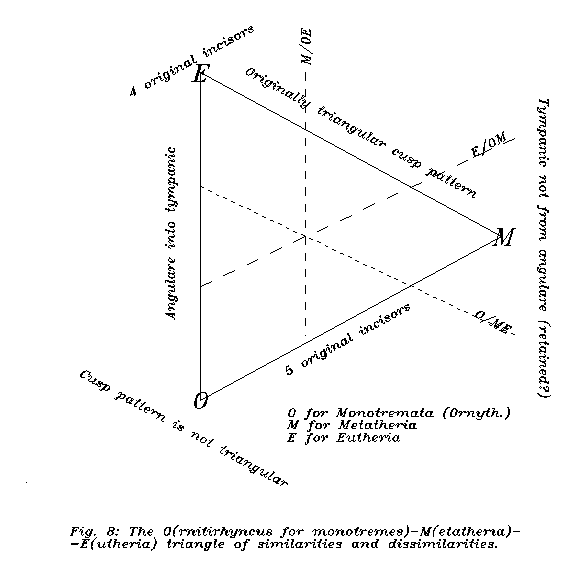
Figure 8: The O(rnithorhynchus for monotremes)-M(etatheria)-
E(utheria) triangle of similarities and dissimilarities.
Now, the problem is: either we can believe in parallel evolution of one of the above characteristics; but we should choose, of which one (and it is generally against the rules of the game to assume independent acquisition of such a characteristic); or there was a trial forking. However in cladistics binary forking is an axiom.
Let us forget about axioms and compare 3 alternative trees, going step by step.
Case 1: first forking into O (Atheria) and M&E (Theria), then the second M/E split. This is suggested by usual taxonomy. Then the triangular cusp pattern is an acquisition between the splits O/M&E and M/E. However then in the O and M&E common ancestor the angulare did not yet form the tympanic (probably the lower yaw still retained it) and later the angulare shifted to the ear region independently in O and E. In addition, the common ancestor of all of them had 5 incisors (no such fossil is known) which was lost in E without any trace.
Case 2: first forking into O&M ("archaic mammals") and E ("advanced ones"). Then the second split is O/M. Then the number of incisors has some explanation: E lost the fifth one in its independent history. Also E could shift the angulare into the ear region: a simple and useful innovation. However then O made the same innovation independently; and what is more, the therian triangular cusp pattern developed twice, independently, once in E and once in M after the O/M split, because the common ancestor cannot have had this "advanced" pattern not present in O: therians would be diphyletic.
Case 3: first forking into O&E ("tympanic mammals") and M; then a second O/E split. The angulare turned into tympanic in the time of O&E common ancestors. But again: then the triangular cusp pattern developed twice, on the independent E and M lineages, and the primitive dentition had 5 incisors: E lost one in early independent life without trace.
In Case 1 the first split should be pushed back before Symmetrodonts, into Early Jurassic or even to Upper Triassic, since Pachygenelus already started to develop a triangular pattern (KEMP 1982). In Case 2 the triagonal therian cusp pattern could not be used at all for cladistics. In Case 3 there would be the same problem with the therian cusp pattern, and the O&E/M split should be very early, because one expects that the fate of the angulare had been determined rather early and no early "mammal" fossil shows any trace of the angulare (KEMP 1982; metatherian fossils are almost recent). And remember that the latest O&M common ancestor was traced back to the J/K boundary. In any of the 3 cases something is fundamentally "against the usual methods of argumentations”.
Triple forkings are indeed extremely rare. A true triangular similarity relation would need two subsequent forkings within the lifetime of the same species, so that changes can be independently combined by crossing. Maybe this happened in the (human, chimpanzee, gorilla) forking where the reconstruction of central fusions is unclear (DUTRILLAUX 1981); after a central fusion a reduced fertility is still retained. However, it would be a very adventurous assumption that the lineages of Eutheria, Metatheria and Monotremata diverged simultaneously from a single species, either on the Tr/J or on the J/C boundary.
Here is the problem stated. It could be solved either by a good absolute chronology of mammals or by a well-established hierarchy of "very fundamental and conservative" characteristics of classical taxonomy.
There would be, of course, the idea of metatherian origin of monotremes (GREGORY 1947) by neotheny. Assume first an E/M split in an early time. The emerging M's (as well as E's) were still fairly primitive especially when very young ("Haeckel's Law"). Then neotheny occurred in a particular M species and appeared an M offshot loaded with many even more primitive ("juvenile and embryonic") characters. To be sure, neotheny occurs and it may have had some part in anthropogenesis (BOLK 1926). But the problem is in the details. One expects such distinctive characteristics in the new O offshot which were not much less archaic in the archaic M's. Now, early M's probably retained the angulare, because their inheritors still not use it as tympanic; then O would have retained it, maybe even together with the articulare. Similarly, the expected O sidewall of braincase would have had a substantial alisphenoid, because advanced cynodonts had such ones (KEMP 1982), while it seems that the reduced alisphenoid is a particular monotreme (and multituberculate) innovation (KERMACK & KIELAN-JAWOROWSKA 1971), which cannot have appeared by neotheny.
Now let us see the dentition in the jaws. In itself the dentition is not too conservative; very various patterns developed during the Tertiary. However, it is believed that the trends are rather irreversible: the reptile ancestors had more numerous teeth and loss of a tooth is much more frequent than the gain. So the reconstructed primitive and "complete" dentitions are believed to be representative, and it is profitable to compare them. Then let us do it, first for the recent groups.
There is consensus about the "complete" therian patterns. For eutherians it is
3 1 4 3
‑‑‑‑‑‑‑ Eutheria
3 1 4 3
Such strange groups as Xenarthra, Pholidotida &c. are not included; for them the primitive dentition is rather unknown. On the other hand, the primitive marsupialian dentition seems to be
5 1 3 4
‑‑‑‑‑‑‑ Metatheria
5 1 3 4
a quite different pattern.
For Monotremata there are problems. Among recent monotremes only Ornythorhynchus anatinus has any dentition, but even that is only juvenile, which is not necessarily representative; and vestigial. Still we have to start with something: the juvenile pattern is
0 1 2 3
‑‑‑‑‑‑‑‑‑ Ornithorhynchus
5 1/2 2 3
(PARKER & HASWELL, 1962).
Now, this pattern is rather peculiar with the many lower incisors and no upper ones, and with the observed second lower canine. However, the lack of upper incisors might be connected with the bill. The second lower canine is often believed to be tooth replacement in progress, but the whole dentition seems juvenile and we shall see examples for more than one canines. Now let us continue for a while.
In the last quarter century teeth of many fossil monotremes have become known (WOODBURNE & TEDFORD 1975, ARCHER et al. 1978, MURRAY 1984, ARCHER et al., 1985, ARCHER et al. 1992; PASCUAL et al. 1992; and citations therein). The fossils do not tell too much about the frontal teeth, but confirm the numbers of premolars and molars. Therefore one may try with the "complete" dentition as
5? 1(2?) 2 3
‑‑‑‑‑‑‑‑‑‑‑‑‑ Monotremata
5 1(2?) 2 3
We can put this reconstructed form to the Upper Cretaceous between, say, Steropodon galmani and Monotrematum sudamericanum, before the ornithorhychid/tachyglossid split.
For the extict "mammal" branches skulls and jaws may help (we use here mainly the drawings from KEMP (1982), SEVERCOV (1981), GÉCZY (1979) and GÉCZY (1989)), although we do not know since when diophyodonty holds (consider the late, if all, appearance of the last molar in present humans), so ±1 errors are possible. In addition, we do not know the "complete" patterns, because of the limited number of fossils. Even then
4? 1 4/5 4/5
‑‑‑‑‑‑‑‑‑‑‑‑‑ Morganucodontia
4/6 1 4/5 4/5
and
4 1 4 7
‑‑‑‑‑‑‑ Pantotheria
4 1 4 7
whence our pattern can easily be derived but the Metatheria pattern probably cannot; and for the suspected sister taxon of monotremes, although as herbivores, their dentition is rather specialised:
1 1 0/3 0/4
‑‑‑‑‑‑‑‑‑‑‑ Multituberculata
1 0 0/3 2/4
in which case the minimal dentition for the hypothetic common ancestor is
5? 1(2?) 3 4
‑‑‑‑‑‑‑‑‑‑‑‑ Multituberculata.-Monotremata. common ancestor?
5 1(2?) 3 4
This common ancestor may have existed because multituberculate and monotreme skull patterns are rather similar (MURRAY 1984) but then it must have lived before Upper Jurassic. (Note that all surviving monotremes are meat-eaters and all known multituberculates were herbivores.) There was then time enough for serious changes. But it seems that for origin we must go back before morganucodontids.
Luckily, advanced cynodont dentitions can be described by the standard mammalian way, and the advanced therapsid tooth replacement mechanism defines the "permanent" pattern with a maximum variation 1 (KEMP 1982). Then we took the skull and jaw reconstructions of Beaumont cited by GÉCZY (1989) and of KEMP (1982) and simply counted the teeth.
Let us see first the suspected sister taxa of "mammals". Tritylodonts were herbivorous and therefore their dentition was special. Indeed,
1 0 0 5
‑‑‑‑‑‑‑ Tritylodon
1 0 0 5
while for ictidosaurs (=tritheledonts) the available older reconstruction gives
2 1 3 8
‑‑‑‑‑‑‑‑ Ictidosauria »Tritheledontia
2 1 0? 8
which is rather poor in incisors, but KEMP (1982) after Crompton reconstructs for Diarthrognathus' lower jaw
? ? ? ?
‑‑‑‑‑‑‑ Diarthrognathus
3 1 4 4
which is almost the primitive therian pattern. For any case tritheledont fossils are poor and fragmented. For more than 4 incisors we must go back to Dvinia and for more than 1 canines to therocephalians (although chiniquodont Probelesodon's dentition can alternatively interpreted as 4 upper incisors and 2 upper canines, a small and a large, or 5 incisors and 1 canines). Of course, some cases with double canines may show transient replacement scenes (KEMP 1982), but the original number of canines was more than 1. Namely, in the early stage of tooth differentiation a canine region appeared where the teeth were enlarged; the location of this region was determined by laws of mechanics (KEMP 1982). It was not necessary to end with a single tooth in this region.
From dentition pattern then monotremes and metatherians seem rather archaic. Comparing this with the results of WESTERMAN & EDWARDS (1992) (and of this paper) the monotreme-marsupial common ancestor must have separated from ours at least as far back as Middle Triassic, while they asundered from each other at the J/C boundary. Such a picture is not self-contradictory, but does contradict to many other data.
Now, finally, let us turn to the individual molar cusp patterns, to distinguish therians and "non-therians". But, because Atheria and Prototheria are often considered synonims, first some considerations are needed for the "goal" of the evolution of the cusp pattern. The result will be that not all non-therians are before the therian grade.
"Goals" are unusual in natural sciences. However there are some examples in physics, where variational principles are often interpreted in such a way that the laws "want" something to be minimal or maximal.
For example, the equations of motion of Newtonian mechanics can be reformulated in the way that along the path of the body an integrated "action" is minimal:
t2
S = ň(K - V)dt = min. (B.1)
t1
where K and V are the kinetic and potential energies, respectively, the path is fixed at the start and end times, t1 and t2. During the French Enlightment period some mathematicians considered this as a signal for the economy of Providence. But very probably this interpretation is physically incorrect (BANAI & LUKÁCS, 1987), since without the equations of motion one could not prescribe the end position, only the initial velocity. But there are "goals", i.e. unidirectional changes in thermodynamics.
In thermodynamics of closed systems a quantity, the entropy, is monotonously growing to a final (asymptotic) equilibrium value which can be calculated from the initial ones "as if it were" the goal.
Animals are open thermodynamic systems, and in the thermodynamics of open systems the correct variational principles are not yet known. However, since the work of GLANSDORFF & PRIGOGINE (1971) it seems that the principle may have the form
{production rate of P} → minimum (B.2)
where P is something akin to entropy. Then the system is going towards the minimal dissipation of something akin to energy. Very loosely speaking the system adapts more and more to the environment.
In microbiology EIGEN (1971) formulated a principle through "survival of fittest". In a competition the winner will be that which propagates the fastest. This means an optimal compromise between abilities and the needed information. If the virus has too much ability, its DNA is too long and it takes too much time to reproduce it; if the ability is too low, the virus reproduces fastly but then dies. Indeed, Spiegelman's minimonsters (EIGEN 1971) demonstrated that the superfluous abilities vanish, when they lost 83% of the original genome in a Paradise-like environment.
Now let us see the molars of advanced cynodonts. They already fitted together and gave good cutting edges but not grinding surfaces. Good cutting needs 2 or rather 3 cusps in a linear arrangement, moving as scissor blades. This stage was reached by advanced cynodonts, morganucodonts and triconodonts. Now, grinding becomes possible and cutting is not endangered if i) the linear arrangement becomes triangular, and in the same time ii) the triangle patterns on the upper and lower molars fit into each other. Then the parallel sides of the triangles cut, and one upper cusp fitting among the lower 3 and vice versa grind. This arrangement is then superior to the linear one, so the linear one is unstable. The trigonal pattern may appear spontaneously at any time. The only potential barrier delaying the transition is the fact that random triangulations, uncorrelated above and below, would result in worse cutting. The trigonal pattern started in Pachygenelus (tritheledont) and it was obvious (either independently or not) in symmetrodonts.
However, observe that now we have compared trigonal (therian) patterns to linear ("pretherian") ones, and not to everything nonlinear. With at least 4 cusps there is an alternative solution for good cutting and grinding. One can form shifted rectangles, paralellepipedons or parallel rows. Then there are 2 cutting edges per molar, and one cusp always fits among 4 of the opposite molar. This pattern is again superior to the linear arrangement, and it is more or less on equal level with the triangular solution. The parallel arrangement seems to have started with docodonts, it was well developed in multituberculates (and in desmostylians).
The morale of this argumentation is that the true evolutionary opposition is not "trigonal" vs. "non-trigonal", i.e. "therian" vs. "non-therian", but rather there is an Y-shaped relation (see Figure 9 as at least a logical possibility). The unevolved "primitive" level is the linear arrangement, which opposes the 2 evolved level, the trigonal and parallel ones, which again oppose each other. By comparing one of the patterns, A, to the other 2, both are "non-A", but that is only a partial information. Multituberculates were not therians, but not pretherians, but a parallel branch.
|
|
Figure 9: A logical possibility for trial opposition of linear, trigonal and quadrangular (or double-row) cusp patterns. Then the therian or triagonal pattern opposes 2 non-therian ones, while the linear primitive pattern opposes 2 nonlinear advanced ones. The advanced patterns are appropriate not only for cutting but for grinding too.
This argumentation does not decide if monotreme molars are very modified trigonals or parallels; that remains to specialists. However it shows that monotreme molars are not pretrigonal living fossil stages from triconodont times, even if they did not change too much since Lower Cretaceous Steropodon galmani.
We have compared the fate of one lower jawbone, the dentition and the molar cusp pattern among the 3 recent mammal groups. Each of the compared characteristics suggests some descent lineages, and all of proposed lineages have good arguments but good counterarguments as well. All of them may have parts of truth, but all have serious problems too. Now let us break the circulus vitiosus of Yes's and No's, and try to turn to a measurement.
APPENDIX C: SOME INPUT DATA OF THE DNA DISTANCE ANALYSIS
WESTERMAN and EDWARDS (1992) give all the data needed for any statistical analysis. For the particular distances they give 2 distances ("to and fro"), the number of measurements, and the standard deviations as follows in Table 3 (let us postpone the last column):
|
|
Pair |
|
|
D |
|
N |
s |
_ |
|
Dcor |
|
|
Oana-Tacu |
|
13.3 |
|
6 |
|
2.3 |
|
13.93 |
|
|
|
|
|
|
11.7 |
|
6 |
|
2.1 |
|
13.26 |
|
|
Oana-Zbru |
|
13.5 |
|
6 |
|
2.5 |
|
14.44 |
|
|
|
|
|
|
14.3 |
|
3 |
|
4.9 |
|
16.21 |
|
|
Oana-Dros |
|
24.2 |
|
5 |
|
2.1 |
|
24.20 |
|
|
|
|
|
|
21.5 |
|
3 |
|
2.1 |
|
24.37 |
|
|
Oana-Meu |
|
23.0 |
|
3 |
|
1.0 |
|
24.77 |
|
|
|
|
|
|
21.9 |
|
3 |
|
2.2 |
|
24.82 |
|
|
Oana-Dvir |
|
23.3 |
|
3 |
|
3.0 |
|
26.24 |
|
|
|
|
|
|
22.8 |
|
2 |
|
1.1 |
|
25.84 |
|
|
Tacu-Zbru |
|
0.06 |
|
8 |
|
0.2 |
|
0.06 |
|
|
|
|
|
|
0.00 |
|
3 |
|
0.0 |
|
0.00 |
|
|
Tacu-Dros |
|
22.7 |
|
2 |
|
0.8 |
|
22.70 |
|
|
|
|
|
|
22.1 |
|
3 |
|
2.1 |
|
23.32 |
|
|
Tacu-Meu |
|
22.2 |
|
4 |
|
1.3 |
|
23.91 |
|
|
|
|
|
|
19.9 |
|
4 |
|
4.7 |
|
21.00 |
|
|
Tacu-Dvir |
|
20.6 |
|
2 |
|
3.8 |
|
23.20 |
|
|
|
|
|
|
23.5 |
|
1 |
|
? |
|
24.80 |
|
|
Zbru-Dros |
|
20.0 |
|
1 |
|
? |
|
20.00 |
|
|
|
|
|
|
18.2 |
|
1 |
|
? |
|
19.47 |
|
|
Zbru-Meu |
|
19.7 |
|
1 |
|
? |
|
21.22 |
|
|
|
|
|
|
22.5 |
|
6 |
|
4.3 |
|
24.07 |
|
|
Zbru-Dvir |
|
22.0 |
|
2 |
|
2.9 |
|
24.78 |
|
|
|
|
|
|
? |
|
0 |
|
? |
|
? |
|
|
Dros-Meu |
|
18.5 |
|
6 |
|
3.2 |
|
19.93 |
|
|
|
|
|
|
18.8 |
|
3 |
|
0.5 |
|
18.80 |
|
|
Dros-Dvir |
|
20.9 |
|
6 |
|
1.6 |
|
23.54 |
|
|
|
|
|
|
23.1 |
|
6 |
|
0.2 |
|
23.10 |
|
|
Meu-Dvir |
|
21.2 |
|
3 |
|
0.7 |
|
23.88 |
|
|
|
|
|
|
20.7 |
|
2 |
|
0.2 |
|
22.30 |
Table 3. The data of the Westerman-Edwards measurement.
Then they performed a correction "for nonrandom measurement errors" and the corrected value is the last column.
Now, the corrections went to "nonrandom errors", so the statistical fluctuations are still there and they did not change for first approximation. Then we, based on the standard methods of statistics, proceed as follows.
For the uncertainty of a particular D the standard formula is
dD = sD/(n-1)1/2 (C.1)
Then we have pairs of D and dD. Since the two data represent the same distance, the final value is the average, weighted as usual by 1/(dD)2, and the final dD is:
dD = {1/(d+D)2 + 1/(d-D)2}-1/2 (C.2)
which is Table 2.
The standard method was impossible in 5 cases, as follows:
1) For Tacu-Zbru the distance is 0 in one direction with d‑D=0. This would automatically set D=0.0±0.0. Instead we averaged by weighting only with the numbers of measurements here.
2) For Tacu-Dvir there is only 1 measurement in one direction, so there the error cannot be calculated. However, there was only 2 measurements in the other direction, so all 3 values could be reconstructed (for the uncorrected at least), and from these 3 data a dD could be calculated. D is calculated from the corrected data.
3) For Zbru-Dros there were 1-1 measurements in each direction, so there was no d±D for the symmetrized average. So we calculated D and dD from the two corrected data with equal weights.
4) For Zbru-Meug error was unavailable in one direction, being a single measurement. D and dD is calculated unilaterally.
5) For Zbru-Dvir there was no measurement in one direction. D and dD is calculated unilaterally.
APPENDIX D: CHROMOSOMAL SEX DETERMINATION IN MONOTREMES
There had been earlier an a priori 50% chance for deciding the mono- or diphyletic origin of recent mammals. If sex determination in monotremes had been of Anas type, then they would have separated from us in non-mammal stage. To see this we list some facts:
1) Human (and therian) sex determination is of Lygaeus type: XX for female, XY for male.
2) Avian sex determination is of Lymantria (or Anas) type: XX for male, XY (or X0) for female (CREW 1965).
3) Intersexes are practically non-reproductive (so select out) for mammals where reproduction needs balanced interaction of sexual hormones.
Let us see these statements in some details. CREW (1965) mentions only 5 non-Lygaeus therians: Apodemus speciosus ainu, Microtus montebelli, Microtus oregoni, Ellobius lutescens and Clethrionomys bedfordiae. But the first 4 cases are doubtful. Namely the first is a subspecies and other variants have the Lygaeus scheme, and in the next 3 ones chromosome fusions appear. So only the fifth case is to be taken seriously, in contrast to an overwhelming majority. Hens certainly have XY chromosomal pattern. Finally some therians are extremely sensitive even to minor influences of the hormones of the opposite sex. A good example is the freemartin heifer, resulted from twin calving with a bull-calf. The female calf is then very frequently sterile. The appearance of these special defective females is so frequent that they were mentioned 2000 years ago (VARRO -36). In that time, it seems, they were not eliminated (for Romans it was imprudent to kill oxen etc. for anything else than sacrifice); and in mature stage a freemartin cow was called taura from taurus while the normal female was vacca: a clear indication for the connection with their bull twins. Another example for the intersex sensitivity of therians is the chalico tomcat. Chalico cats are by rule female, since the colour combination is possible only with two X chromosomes. However rarely the same colour appears on tomcats (an explanation is XY/XXY mosaicism); but they are all sterile.
On the other hand intersex series are quite common in classes Pisces and Amphibia, e. g. in Rana sylvatica the sex state depends on environmental influences during maturation and a whole series from strong males to strong females can be produced (WITSCHI 1929). Clear chromosomal sex determination is rare even among reptiles; Crew can mention only some venomous snakes plus Vipera berus (which is viviparous, so has special female role). Even birds, where there is chromosomal sex determination, are much easier to be sex-inverted than mammals. By eliminating the operative left ovarium of hens, the inoperative and vestigial right ovarium starts into a testis and in some cases the resulting cock is reproductive (CREW 1965): a perfect inversion not reported for any normal mammal.
Then one can conclude that therians must have chromosomal sex determination, and for monophyletic mammals the determination must be of Lygaeus type. Furthermore, the difference between therian and avian determination plus the lack of chromosomal determination in most recent reptiles mean that the common ancestor had no chromosomal sex determination at all, so sexual differences were small and intersexes reproductive in Lower Carboniferous, i.e. mammal (and avian) chromosomal sex determination is probably younger than that of insects (and the dimorphism is smaller, too).
Now, in the second half of 40's some Australian biologists (Matthey and White, at least) reported (with some doubt) Lymantria or Anas type sex determination for monotremes (CREW, 1965). Were it true, it would be an indication that monotremes separated from us at a non-mammal grade.
However, recent data show that monotreme sex determination is also of Lygaeus type albeit with serious modifications. First, the therian female chromosome X is replaced by 2 ones, X1 and X2 (BICK 1992). Such a phenomenon is unknown among therians (although its analogy with two Y's is reported for 2 eutherians, Sorex araneus and Gerbillus gerbillus, and for 2 metatherians, Wallabia bicolor and Potorous tridactylus (CREW 1965) while can be observed in genera Mantis and Hierodula of orthopterans. Second, there is a complicated meiotic chain of chromosomes in monotremes, including the sex chromosomes (BICK 1992) but probably some others too.
There is a natural way to interpret the chain. According to WHITE (1957), sex chromosomes evolved from autosomes by collecting the sexually determinative factors onto a single pair of chromosomes. The process has not reached perfection even in therians: on one hand the X chromosome still contains such sexually neutral genes as for colour vision, on the other hand there are human intersexes with normal chromosome constitution. We mention only 2 examples. The ovarian hypoplasy syndrome appears with a normal female XX, but the ovary does not work correctly; in this case the source is autosomal (LÁSZLÓ et al. 1976). In testicular feminisation an XY kariotype and working testis is accompanied with a generally female constitution; but the body cannot accept the testosteron, probably because a defect in 5-a-reductase, whose gene may or may not be on the X chromosome but very probably not on the "appropriate" male Y. Still, the sex chromosome pair evolved quite far in therians.
Sex differences are smaller in monotremes than in therians. The number of milk glands do not differ in males and females. GRZYMEK (1966) even reports a male tachyglossid from the Prague zoo which started to develop the transient pouch in each 28th day, i. e. had some ovulation cycle. Still, differences are serious between the normal male and female modes.
The common Lygaeus sex determination does not rule out separate origins of therians and monotremes from different therapsid reptiles, especially if cynodont mothers performed clearly female functions about children (MURRAY 1984). If so, then intersex individuals were not welcome by evolution even at the advanced cynodont stage, although the tolerance may have been greater than now. In therians the low frequency of intersexes is guaranteed by collecting all sexual genes to "male" (Y) and "female" (X) chromosomes, through crossovers, fusions, translocations etc. Now the meiotic chain may be the first step in collecting all sexually important features together (WRIGLEY & GRAVES 1988), to avoid intersexes as far as possible. In a meiotic chain the recombination of parental chromosomes in the chain is impossible (a well known example is Oenothera lamarckiana (RENNER 1925)), and then the sexually important genes remain together; the whole meiotic chain behaves as a single chromosome, restricting recombinations (but not crossover) for the chromosomes involved.
We note that there exist some theories suggesting that sex chromosome evolution goes towards the loss of Y (WHITE 1957). However, this did not happen in mammals, while it is frequent in insects. But, it seems, insect chromosomal sex determination is older.
REFERENCES
ÁBRAHÁM, A. (1964): Összehasonlító állatszervezettan, Tankönyvkiadó, Budapest (in Hungarian)
ARCHER, M., PLANE, M. D. & PLEDGE, N. S., (1978): Additional evidence for interpreting the Miocene Obdurodon insignis etc. The Aust. Zool. 20, 9-27
ARCHER M. & CLAYTON G. (eds.) (1984): Vertebrate Zoogeography. Evolution in Australasia. Hesperian Press, Merrickville
ARCHER, M. et al., (1985): First mesozoic mammal from Australia - an Early Cretaceous monotreme. Nature, 318, 363-366
ARCHER, M. et al. (1992): Description of the skull and non-vestigial dentition of a miocene platypus etc. in AUGEE, M. L. (ed.), Platypus and echidnas, Beaver Press, Strawberry Hills, p. 15-27
AUGEE, M. (1984): Quills and bills etc. in ARCHER, M. & CLAYTON, G., Vertebrate zoogeography. Evolution in Australasia. Hesperian Press, Merrickville, p. 567-570
BANAI, M. & LUKÁCS, B. (1987): Variációs elvekről és mozgásegyenletekről. Fiz. Szemle 37, 337-342
BÉRCZI Sz. & LUKÁCS B. (1998): Some notes to the Permotriassic climate implications from mammalogenesis. Acta Climatologica 31A, 37-50
BICZÓ, G., LADIK, J. & GERGELY, J. (1966): Approximate calculation of the tunneling frequencies of the proton in the N-H...O Hydrogen bond of the nucleotide base pairs. Acta Phys. Hung. 20, 11-24
BOLK, L. (1926): Das Problem der Menschwerdung. Jena
BUDYKO, M. I., RONOV, A. B. & YANSHIN, A. L. (1987): History of the Earth's atmosphere. Springer, Berlin
CLEMENS, W. A. et al. (1989): Biogeography and phylogeny of the metatheria, in WALTON, D. W. & RICHARDSON, B. J. (eds.), Fauna of Australia, Vol. 1B, Mammalia, Austr. Government Publ. Serv., Canberra, p. 527-548
CREW, F. A. E. (1965): Sex-Determination. Methuen, London
CROMPTON A. W. & LUO Z. (1993): The relationships of the Liassic mammals Sinoconodon, Morganucodon ehleri and Dinnetherium, p. 30-44 in Mammal Phylogeny, ed. by. SZALAY F. S. & al., Springer, New York
DUTRILLAUX B. (1981): Les chromosomes des primates. La Recherche, 12, 1247
EDMEADES, R. & BAUDINETTE, R. V. (1975): Energetics of locomotion in a monotreme the echidna Tachyglossus aculeatus. Experientia 31, 935-936
EIGEN, M. (1971): Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften, 58, 465-523
EISENHARDT, L. P. (1950): Riemannian geometry. Princeton Univ.Press, Princeton
ERWIN, D. H. (1993): The Great Paleozoic crisis. Columbia Univ. Press, New York
FARQUHAR, R. M. (1967): Radioactive geochronology, in Runcorn, S. K. (ed.), International dictionary of geophysics, Pergamon Press, Oxford
FOWLER, W. A. (1972): What cooks with solar neutrinos? Nature 238, 24-26
FRASER ROBERTS, J. A. (1967): An introduction to medical genetics. Oxford University Press, London
GÉCZY, B. (1979): Ősállattan. Tankönyvkiadó, Budapest
GÉCZY, B. (1989): Őslénytan. Tankönyvkiadó, Budapest
GÉCZY, B. (1996): Extinctions and astroblemes. Annales Univ. Sci. Budapestiensis, Sect. Geophysica & Meteorologica 12, 13-16
GLANSDORFF, P. & PRIGOGINE, I. (1971): Thermodynamic theory of structure, stability and fluctuations. Wiley Interscience, New York
GREGORY, W. K. (1947): The monotremes and the palimpsest theory. Bull. Amer. Mus. Nat. Hist. 88, 1-52
GRIFFITHS, M. (1989): Tachyglossidae, in WALTON, D. H. & RICHARDSON, B. J. (eds.): Fauna of Australia, Vol. 1B, Mammalia. Australian Government Publishing Service, Canberra
GRZYMEK, B. (1966): Vierfüssige Australier. Kindler, Munich
GUIDE BOOKLET (1992): for the Museum of Geological Survey of Japan, Tsukuba, Japan
HUEHNE von, F. R. (1956): Paläontologie und Phylogenie der Niederen Tetrapoden. VEB Gustav Fischer Verlag, Jena
ISOZAKI, Y. (1994): Superanoxia across the Permian-Triassic boundary etc. Pangea: Global environments and resources. Canadian Society of Petroleum Geologists, Memoir 17, 805- 812
ISHIDA, K., YAMASHITA, M. & ISHIGA, H. (1992): P/T boundary in pelagic sediments in the Tanba Belt, Southwest Japan. Geol. Rept. Shimane Univ. 11, 39-57
JÁNOSSY, L. (1965): Theory and practice of evaluating measurements. Oxford University Press, Oxford
JENKINS, F. A. Jr. & SCHAFF, C. R. (1988): The Early Cretaceous mammal Gobinocodon (Mammalia, Triconodonta) from the Cloverly Formation in Montana. J. Vert. Paleont. 8, 1-24
KEMP, T. S. (1982): Mammal-like reptiles and the origin of the mammals. Academic Press, London
KERMACK, K. & KIELAN-JAWOROWSKA, Z. (1971): Therian and non-therian mammals, in KERMACK, D. & KERMACK, K. (eds.), Early mammals, Academic Press, London
KIELAN-JAWOROWSKA Z. & al. (1987a): The origin of egg-laying mammals. Nature 326, 871-873
KIELAN-JAWOROWSKA Z. & al. (1987b): Early Cretaceous multituberculates from Mongolia and a comparison with Late Jurassic forms. Acta Palaeont. Pol. 32, 3-47
KIELAN-JAWOROWSKA Z. (1997): Characters of multituberculates neglected in phylogenetic analyses of early mammals, Lethaia 29, 249-266
KRAUSE, D. E. & BONAPARTE, J. F. (1990): The Gondwanatheria, a new suborder of Multituberculata from South America. J. Vert. Paleont. 10, 31A
KREBS B. (1991): Das Skelett von Henkelotherium guimarotae gen. et spec. nov. (Eupantotheria, Mammalia) aus dem Oberen Jura von Portugal. Berliner geowiss. Abh. 133, 1-121
LÁSZLÓ, J., GAÁL, M. & BŐSZE, P. (1976): Cytogenetical investigations in ovarian hypoplasia. Clin.Genet. 9, 61-70
LILLEGRAVEN, J. A. & KRUSAT, G. (1991): Cranio-manduibular anatomy of Haldanodon exspectatus (Docodonta, Mammalia) from the Late Jurassic of Portugal and its implications to the evolution of mammalian characters. Contr. Geol. Univ. Wyoming 28, 39-138
LUO Z. (1994): Sister taxon relationships of mammals and the transformations of the diagnostic mammal characters. p. 98-128 in In the Shadow of Dinosaurs, ed. by FRASER N. C. & SUES H. D., Cambridge University Press, Cambridge
LUO Z., CROMPTON A. W. & LUCAS S. G. (1995): Evolutionary origins of the mammalian promontorium and cochlea. J. Verteb. Paleont. 15, 113-121
MIONO SH. (1996): Astronomical significance of microspherule recovered from Palezoic and Mesozoic radiolarian chert in Japan, p. 23-33 in Proceedings of the international meeting Spherules and Global Events, ed. by Detre CS. H. & al., KFKI-1996-05
MIONO SH. (1998): Studies on microspherules in the Permo-Triassic, p. 15-20 in Impact and extraterrestrial spherules. Ed. by the Japan IGCP Committee, Tokyo
MURRAY, P. (1984): Furry egg-layers: The monotreme radiation, in ARCHER, M. & CLAYTON, G. Vertebrate zoogeography. Evolution in Australasia. Hesperian Press, Merrickville, p. 567-570
PARKER, T. J. & HASWELL, W. A. (1962): A text-book of zoology. Macmillan and C°, London
PASCUAL R. et al. (1992): The first non-Austral monotreme etc. in AUGEE, M. L. (ed.), Platypus and echidnas, Beaver Press, Strawberry Hills, p. 1-14
PASCUAL R. & al. (1999): The first gnathic remains of Sudamerica: Implications for Gondwanathere relationships. J. Verteb. Paleont. 19, 373-382
PIMENTEL, G. C. & MCLELLAN, A. L. (1960): The Hidrogen bond. Freeman, San Francisco
QUIROGA, J. C. (1980): The brain of the mammal-like reptile Probainognathus jenseni (Therapsida, Cynodontia) etc. J. für Hirnforschung 21, 299-336
RENNER, O. (1925): Untersuchungen über die faktorielle Konstitution einiger Komplex-heterozygotischer Oenotheren. Bibl. genetica 9, 1-168
SEVERCOV, A. S. (1981): Vvedenie v teoriyu ëvolyucii. Izd. Moskogo Universiteta, Moscow
SCILLATO-YANÉ, G. J. & PASCUAL, R. (1985): Un peculiar Xenarthra del Paleoceno de Patagonica (Argentina) &c. Amenghiniana 21, 173-176
TÓTH I. et al. (1997): A possible nearby supernova explosion in the Permo-Triassic boundary etc. TISS, ed. by MIURA, Y. Yamaguchi University, pp. 18-19
VARRO, M. T., (-36): Rerum rusticarum libri tres.
WALTON, D. H. & RICHARDSON, B. J. (eds.) (1989): Fauna of Australia, Vol. 1B, Mammalia. Australian Government Publishing Service, Canberra
WESTERMAN, M. & EDWARDS, D. (1991): The divergence between echidna and platypus etc., Aust. Mamm. 14, 115-120
WESTERMAN, M. & EDWARDS, D. (1992): DNA hybridization and the phylogeny of monotremes, in AUGEE, M. L. (ed.), Platypus and Echidnas, Beaver Press, Strawberry Hills, p. 28-34
WHITE, M. J. D. (1957): Chromosomal evolution and speciation. Survey of Biol. Progress 3, 109-147
WITSCHI, E. (1929): Studies on sex differentation and sex determination in amphibians. J. Exp. Zool. 54, 157-223
WOODBURNE, M. O. & TEDFORD, R. H. (1975): The first tertiary monotreme from Australia. Amer. Mus. Nov. 2588
WRIGLEY, J. M. & GRAVES, J. A. M. (1988): Sex chromosome homology and incomplete tissue-specific X-inactivation suggest that monotremes represent an intermediate stage of mammalian sex chromosome evolution. J. Hered. 79, 115-118
**********************
Notes added in 2005:
A: Already the consensus is not so strong. E.g. one author mentions as many as 10 reptile subclasses with tricky modifications of aperture structures. Or, some authors call (colloquially) Archosauria Triapsids. But these details are of secondary importance now. Still it seems that Subclass Synapsida is stable with no transitions.
B: No. Grade Reptilia is no more monophyletic. When the ancestors of present mammals and present birds ceased to be amphibians, the two divergent lines were already established (although it seems the respective reptiles were still similar). See e.g. S. Kumar & S. B. Hedges: A molecular timescale for vertebrate evolution. Nature 392, 917-20 (1998).
C: In due course you will met an example of such a “trilobal” classification. If you accept the mammal definition of D/Sg articulation… We should have made a “predictory” note in 1998. The example is in the second chapter upwards in the text!
D: We should have written here some intelligent sentences about actions of Hox genes. I did it, however, elsewhere, see my On the Emergence of Mammals at http://www.rmki.kfki.hu/~lukacs/adelobasil.html.
E: I recently told this to a lady biologist who was rather sceptic. But the original argument came from our lady physicist and I still accept the argument.